Introduction
Autoimmune progesterone dermatitis (APD) is a rare, cyclical, and mucocutaneous hypersensitivity reaction to peak levels of endogenous progesterone seen in women, in the luteal phase of the menstrual cycle. It is an underdiagnosed, complex disease associated with high morbidity1. Therefore, recognition of this process is important as it can result in significant quality of life impairment among women.
Our case report describes one of the rare cases of APD, manifesting as urticarial lesions.
Clinical case
We describe the case of a 41-year-old female patient, previously healthy, with no history of atopic or allergic dermatitis. She was not taking any medication and there was no history of atopic or allergic contact dermatitis.
The patient presented to the dermatology department with a 3-year history of a monthly relapsing pruritic eruption. The patient stated these lesions usually appeared 5-7 days before the onset of menses and resolved approximately 3-4 days after menstruation. She had been previously treated with antihistamines and oral corticosteroids with only temporary relief.
She had had no symptoms during her two pregnancies and she used an intrauterine copper device as a birth control method. There was no history of dysmenorrhea, menstrual irregularities, or oral contraceptive use.
On examination, during the luteal phase, the patient presented multiple maculopapular pruritic non-evanescent wheals distributed symmetrically throughout the body, lasting for more than 24 h, compatible with urticarial lesions.
Several laboratory studies were performed and were all normal or negative, including: ANA, anti-DNA, Sm, SSa, SSb, and RNP antibodies; C3, C4, CH100, and C1-inhibitor; thyroid stimulating hormone, T3, and T4; immunoglobulin G, immunoglobulin A, immunoglobulin M, and immunoglobulin E (IgE).
Hormonal analysis collected at day 3 and day 21 of the menstrual cycle revealed progesterone and estradiol serum levels were also normal during the follicular (1.57 ng/mL and 49.8 pg/mL, respectively) and the luteal phase (2.65 ng/mL and 64.3 pg/mL, respectively).
With the suspicion of APD an intradermal test with 0.1 mL of an 150 mg/mL aqueous solution of medroxyprogesterone serially diluted with 0.9% sodium chloride to concentrations of 0.1 and 10 mg/mL was performed on the 7th day of the cycle. A positive reaction was observed within 2 h, remaining positive for about 24 h (Figs. 1 and 2).
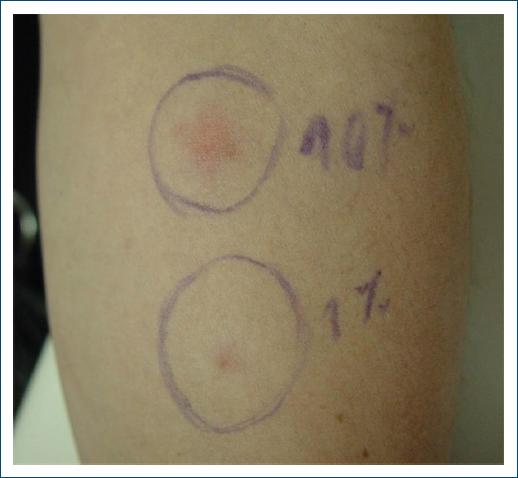
Figure 1 Intradermal test with aqueous solution of medroxyprogesterone (concentrations of 0.1 and 10 mg/mL) 2 h after.
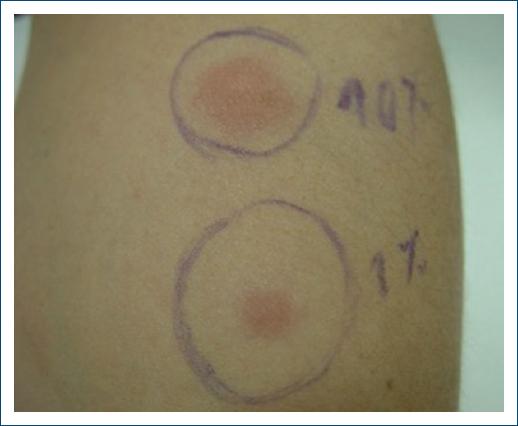
Figure 2 Intradermal test with aqueous solution of medroxyprogesterone (concentrations of 0.1 and 10 mg/mL) 5 h after.
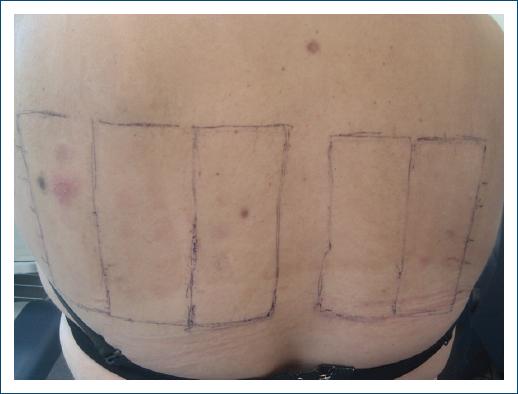
Patch tests were also performed, with the same medroxyprogesterone solution, the standard series of the Portuguese Contact Dermatitis Group, corticosteroids series, and metal series, which revealed positive reactions only to nickel sulfate 5% pet (++) and palladium chloride 1% pet (+) (Fig. 3).
The diagnosis of APD was made and the patient was treated with tamoxifen 40 mg/day, with almost complete clinical clearing. Four months after the dose was reduced to 20 mg/day, with no relapsing and subsequently to 10 mg/day. Six months later, the patient remains free of symptoms and with no side effects.
Discussion
APD is an extremely rare disease, characterized by recurring dermatologic manifestations during the luteal phase of the menstrual cycle2. APD has also been reported to be triggered by exogenous progesterone exposure or pregnancy, with peripartum onset and flares in a subset of patients3. In this case, the patient had no symptoms while she was pregnant.
The cause of APD is not known. It seems exogenous progesterone exposure, such as those used for oral contraception pills or in vitro fertilization, is an important cause of morbidity and may stimulate the body to form progesterone-specific IgE antibodies, that cross-link activate mast cells resulting in APD4. However, not all patients with this disease have a history of exposure to exogenous progesterone, as in the case we just described.
APD can have many different presentations including recurrent and cyclical urticaria with or without angioedema, anaphylaxis, pruritus, and dermatitis. Other presentations include vesiculobullous disorders, erythema multiforme, fixed drug eruptions, aphthous stomatitis, maculopapular rash, and recalcitrant dermatitis5-7. The lesions are characteristically symmetrical and occur on the face, trunk, and extremities. Our patient presented with urticarial lesions, which is one of the most common manifestations of this dermatitis.
The mean age at the beginning of symptoms is 27.3 years6. Symptoms usually appear 3-4 days before menstruation when progesterone levels peak and resolve within a few days after the onset of menstruation as progesterone levels reduce, only to recur just before the next period1, as in this case.
Diagnosis is difficult and often delayed. It is frequently made based on the exclusion of all possible differential diagnoses.
The diagnostic criteria for APD proposed by Warin are: skin lesions related to menstrual cycle; symptomatic improvement after inhibiting progesterone secretion by suppressing ovulation; positive response to intradermal testing with progesterone8. Intradermal progesterone tests may be used to help diagnose APD; however, the test is not standardized, has unknown sensitivity and specificity, and test results do not typically change management. In a series of 24 cases of APD, only 50% of patients showed a positive result to this test. In patients presenting with urticaria and/or anaphylaxis, the intradermal skin test may potentially be of more value9. In this case, we performed an intradermal test with an aqueous solution of medroxyprogesterone at concentrations of 0.1 and 10 mg/mL on the 7th day of the menstrual cycle, and it was positive.
In regards to treatment, primary treatment includes prescribing a combination of oral contraceptives. Other successful treatment options include gonadotropin-releasing hormone agonists, danazol and tamoxifen; topical and oral antihistamines and steroids to treat cutaneous symptoms; and bilateral oophorectomy in patients experiencing persistent symptoms2. Here, the patient was treated with tamoxifen with complete resolution of symptoms.