Introduction
Skin ulcers are a highly prevalent health issue, mostly in elderly patients, and lead to healthcare costs estimated in over 20 billion dollars per year in the United States1. They are characterized by loss of continuity affecting epidermis and dermis, which may also affect deeper tissues, such as subcutaneous, fascia, muscles, and tendons and may expose blood vessels, bones, and joints. On an acute loss of continuity, there is a stimulus for the beginning of the healing process, which is divided in three phases: inflammatory, proliferative, and remodeling. If, however, there is interference in the physiologic healing process – especially during the inflammatory phase – the lesion will become a chronic one. Chronic ulcers are those with no signs of healing stimulus after at least 4 weeks, persisting stationed within the inflammatory phase of healing2,3.
A chronic ulcer brings significant morbidity and may affect in many ways the patient's quality of life, in aspects ranging from malodor, exudate draining, need for frequent dressing changes, and compromised mobility, to the increase in the number of hospital admissions up to the need for amputation4,5. It also brings an increase in mortality rate, usually due to secondary infections with subsequent systemic involvement. For instance, diabetic ulcers have a mortality rate in 5 years of up to 30.5%3,6,7, similar to the one found in cancer (31%)8.
Factors that may negatively affect the healing of a skin ulcer, leading to chronification, can be divided between local and systemic factors. Among local factors are tissue oxygenation, infection or critical colonization of the wound, biofilm formation, and the possibility of infection in adjoining tissues. Several systemic factors are reported to influence in the healing process: sustained hyperglycemia in diabetes mellitus (DM), for instance, which delays healing by increasing reactive oxygen species and advanced glycation end products, besides causing angiopathic and neuropathic complications9,10. Obesity also dysregulates the systemic inflammatory response, increasing the risk of healing complications. Alcoholism may impair the proliferative phase of healing by reducing angiogenesis. Smoking leads to tissue hypoxia due to toxic effects of nicotine, carbon monoxide, and hydrogen cyanide, among other mechanisms. Some drugs also negatively affect healing. For instance, glucocorticoids may harm fibroblast proliferation and the cell defense response, augmenting the risks of local infection. Non-steroidal anti-inflammatory drugs decrease angiogenesis, and chemotherapic drugs hinder cell proliferation11.
Nutrition is another very important factor for a healthy healing. Proteins are essential to collagen production, angiogenesis, and tissue regeneration, and a hyperproteic diet may be indicated12. Supplementation of amino acids such as arginine and glutamine may benefit healing13,14. Adequate intake of fluids, calories, fatty acids, vitamins, and micronutrients such as magnesium and zinc is also of utmost importance for healing. Protein-caloric malnutrition is, therefore, a frequent and underdiagnosed cause of non-progression in wound healing14,15.
The lower extremity (LE) is the most frequent site of a chronic ulcer – especially in the distal portions of the leg and in the foot. In developed countries, the most common etiology of a chronic lower-extremity ulcer (CLEU) is chronic venous insufficiency (CVU), which comprises up to 80% of cases. Peripheral artery disease, either isolated or in association to CVU, is present in up to 25% of CLEU located in legs16. Non-controlled systemic arterial hypertension (SAH) and inflammatory diseases such as pyoderma gangrenosum are among other less frequent causes17,18. Foot ulcers are usually related to some degree of neuropathy (autonomic, sensitive, or motor), with consequential impairment of protective mechanisms of skin barrier, and biomechanical alterations in gait, leading to repeated traumas and smaller regenerative capacity. In developed countries, this neuropathy is most commonly associated to DM16. Pressure injuries are another frequent cause of skin ulcers in inpatients and those with impaired mobility12.
Although there are many studies regarding the clinical and epidemiological profile of patients with CLEU, most of them come from developed countries, mainly in Europe and North America. These countries have already been through the epidemiologic transition, when improved life conditions resulted in decreased morbimortality by transmittable diseases, but with an elevation in the rate of degenerative chronic diseases, due to enhanced life expectancy. This scenario may vary considerably in other regions of the world. Besides that, socioeconomic status of the individual and of the population seems to also affect healing4. In the least developed or developing countries, the main causes of CLEU are infectious and neoplastic19,20. There are few studies about the clinical and epidemiological profile of CLEU patients in Brazil, a country characterized by a double burden of diseases: while better life expectancy has led to an increase in chronic diseases such as DM and vascular disease, there is still a high prevalence of infectious diseases and of food insecurity resulting in malnutrition21-24. Public health problems unfortunately keep Brazil as the second country in the world regarding the number of leprosy cases, a disease which is a common cause of peripheral neuropathy and that may contribute to development of ulcers25.
The objective of this study is to characterize the clinical and epidemiological profile of outpatients with CLEU, treated in two reference hospitals of the public health system in the state of Paraná, in the Southern region of Brazil, and to search for factors that may be related to the severity of these ulcers.
Methods
This is an analytic and cross-sectional study, which evaluated 68 patients with CLEU, totaling 109 lesions, in public health-care outpatient clinics of two tertiary hospitals in the Brazilian state of Paraná: Hospital Santa Casa de Curitiba, in the city of Curitiba, and Hospital de Dermatologia Sanitária do Paraná, in the city of Piraquara (a city in Curitiba metropolitan area).
Patients older than 18 years having LE ulcers for more than 4 weeks, presenting for medical visit or for dressing changes in the participating clinics, were invited to take part in the study, and data collection was performed during the period of June 2021-February 2022.
During the initial assessment, data were collected on demographic (age, sex, and municipality of residence) and clinical (including information on comorbidities, drugs used, and risk factors such as smoking and alcoholism) characteristics. Anthropometric measures of height and weight were also performed, to allow calculation of body mass index (BMI). In regard to BMI, patients were classified in groups of nutritional status according to the World Health Organization (WHO) cut offs: grades 1-3 underweight, normal weight, overweight, or grades 1-3 obesity.
In relation to CLEU, data were collected on etiology (as assessed by attending physicians and registered in medical records), characterizing as venous, arterial, diabetic, neuropathic ulcer of leprosy, pressure injury, or post-traumatic. It was also noted presence or absence of lymphoedema as an etiologic factor.
Considering the number of lesions, patients were divided in three groups: those with one, two or with three or more wounds. Wounds were divided according to their anatomic location, as proposed by Baker et al.26, as located in proximal LE (that is, located in the larger calf circumference or proximal to it), in distal leg, or in the foot. Moreover, duration (in years since the onset of ulcer) and size (calculated by multiplying the largest longitudinal and transverse axes of each ulcer) were noted. The size was measured and registered with validated, sterilizable, and flexible rulers2, during dressing changes.
Data were stored in Microsoft Office Excel® (2019 version). Results of quantitative variables were described by mean, standard deviation, median, minimum, and maximum. Categorical variables were described by absolute and percentage frequencies.
Besides the descriptive analysis, it was verified if duration, size, or number of CLEU per patient would potentially be related to any aforementioned variable. To assess the association between two categorical variables, the Pearson's Chi-squared test was performed. To compare the duration of wound among groups defined by classification of categorical variables, nonparametric Mann-Whitney or Kruskal-Wallis tests were performed. To compare groups defined by the number of lesions (with one, two, or at least three wounds) in relation to quantitative variables, the analysis of variance model or nonparametric Kruskal-Wallis test was performed. To analyze the correlation between two quantitative variables, Spearman correlation coefficients were estimated. Normality assumption of continuous quantitative variables was performed by Kolmogorov-Smirnov test. Values of p < 0.05 indicated statistical significance. Data were organized in an Excel® sheet and analyzed with IBM SPSS Statistics software (v.20.0. Armonk, NY: IBM Corp).
Approval for the study was granted by the Ethics in Research Committee of Federal University of Paraná (CAAE 44216921.8.0000.0102, approval number 4.649.628). Both participating hospitals also provided consent to performing the research in their outpatient clinics. An informed consent term was provided for all participant patients to sign.
Results
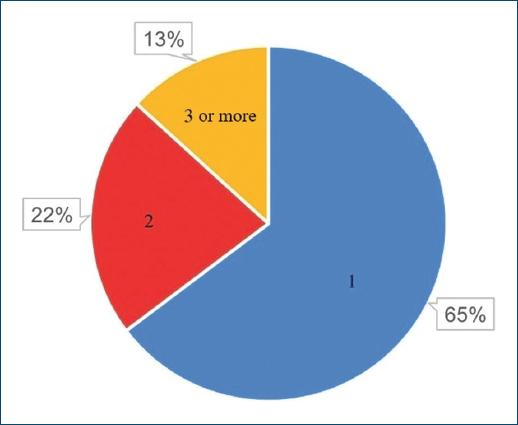
A total of 68 patients took part in the study: 40 (58.82%) and 28 (41.18%) females. As patients presented a mean number of 1.6 CLEU each, a total of 109 wounds were recorded. Further data on age, BMI, duration of ulcer, number of wounds per patient, and size of largest wound are presented in table 1. The most common age group was 60-79 years old (Fig. 1), corresponding to 43 (57.35%) patients. Forty-three (63.24%) patients presented with only one CLEU (Fig. 2). Only 1 (1.59%) patient had a CLEU larger than 500 cm² (Fig. 3). Most patients were in BMI groups classified as overweight (30.88%) or obese (33.82%), as shown in figure 4.
Table 1 Clinic and nutritional characteristics of CLEU patients
| Variable | Mean ± standard deviation | Median (minimum-maximum) |
|---|---|---|
| Age (years) | 65.1 ± 13.6 | 66 (16-91) |
| BMI (kg/m²) | 28 ± 6.4 | 27.8 (13.7-45.9) |
| Duration (years) | 9.7 ± 12.2 | 5.5 (0.12-50) |
| Number of wounds | 1.6 ± 0.98 | 1 (1-5) |
| Size of larger wound (cm2) | 80 ± 140 | 30 (0.25-900) |
CLEU: chronic lower extremity ulcers; BMI: body mass index.
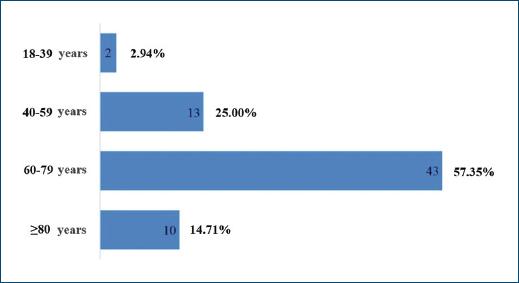
Figure 1 Distribution of CLEU patients according to age group. CLEU: chronic lower extremity ulcers.
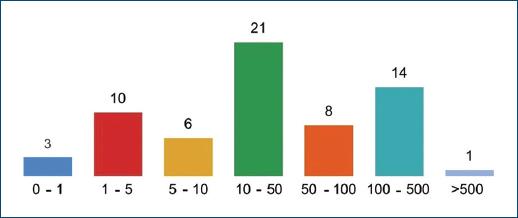
Figure 3 Distribution of CLEU patients according to size of larger wound, in cm². CLEU: chronic lower extremity ulcers.
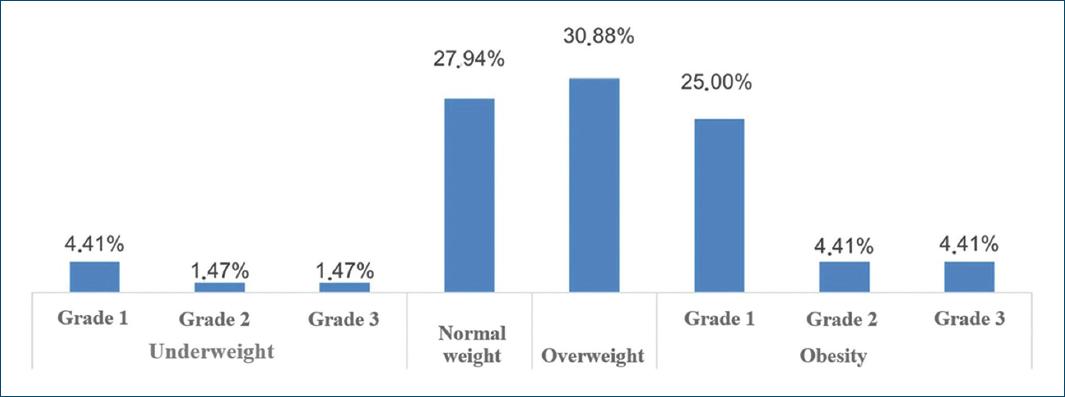
Figure 4 Distribution of CLEU patients according to WHO classification for BMI. CLEU: chronic lower extremity ulcers; WHO: World Health Organization; BMI: body mass index.
Seven (10.29%) patients were assessed in Hospital Santa Casa de Curitiba, and the other 61 (89.71%) in Hospital de Dermatologia Sanitária do Paraná. Patients came from 16 different municipalities, with 6 (8.82%) patients from Curitiba and the remainder from municipalities of Curitiba metropolitan area. The municipality with the most share of patients in the study was Piraquara, with 29 (42.65%) patients.
History of smoking and of alcoholism were present in 33 (48.53%) and 12 (17.65%) patients, respectively, and 13 (19.12%) patients were wheelchair users. Main etiology of CLEU was venous - in 39 (57.35%) patients (Fig. 5). There was lymphoedema in 7 (10.29%) patients, five of which had primarily venous ulcers (12.82% out of the 39 patients with venous ulcers), and two which had neuropathic leprosy ulcers (25.00% out of the eight patients with neuropathic leprosy ulcers). The most common anatomical location of CLEU was the distal leg, representing 49 (44.96%) out of the 109 assessed lesions (Fig. 6).
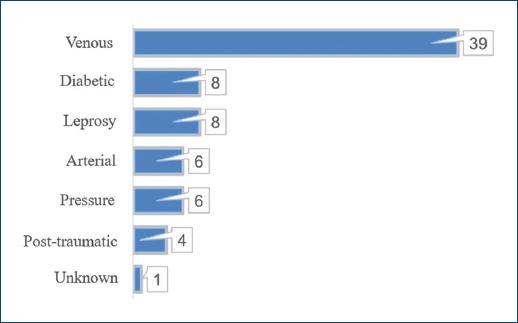
Figure 5 Distribution of CLEU patients according to wound etiology. CLEU: chronic lower extremity ulcers.
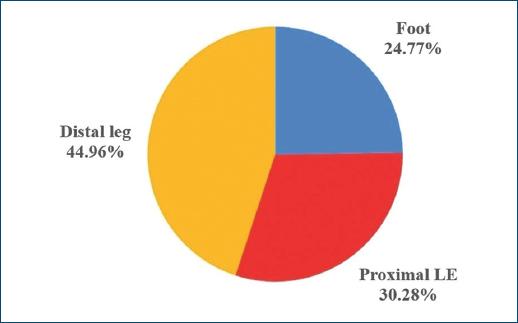
Figure 6 Site of CLEU, according to LE anatomic region. CLEU: chronic lower extremity ulcers; LE: lower extremity.
Duration of a CLEU was associated, with statistical significance (p = 0.027), to a larger wound size (Table 2). The duration had a statistically significant association also with some etiologies (Table 3): diabetic ulcers had a shorter duration (p = 0.003), while venous ulcers and those with lymphoedema had a longer duration (p = 0.002 and p < 0.001, respectively).
Table 2 Correlation of quantitative variables to duration of CLEU and to size of largest wound
| Duration time | Correlation coefficient* | p† |
|---|---|---|
| Age | −0.11 | 0.401 |
| BMI | 0.16 | 0.195 |
| Size of largest wound | 0.28 | 0.027 |
| Number of wounds | 0.20 | 0.122 |
| Size of largest wound | Correlation coefficient* | p† |
| Age | 0.06 | 0.630 |
| BMI | 0.22 | 0.077 |
| Number of wounds | 0.20 | 0.109 |
*Spearman correlation coefficient.
†p-values obtained from Kolmogorov-Smirnov test. CLEU: chronic lower extremity ulcers; BMI: body mass index.
Table 3 Association of duration time of CLEU to etiology and comorbidities
| Duration time (years) | |||
|---|---|---|---|
| Aetiology | Mean ± SD | Median (minimum-maximum) | p* |
| Venous | |||
| No | 5.7 ± 9 | 1 (0.1-33) | 0.002 |
| Yes | 13.1 ± 13.6 | 8 (0.1-50) | |
| Diabetic | |||
| No | 11 ± 12.6 | 6.5 (0.1-50) | 0.003 |
| Yes | 0.9 ± 1 | 0.5 (0.2-3) | |
| Arterial | |||
| No | 10.4 ± 12.5 | 6 (0.1-50) | 0.183 |
| Yes | 2 ± 1.8 | 2 (0.3-5) | |
| Pressure injuries | |||
| No | 10.2 ± 12.6 | 6 (0.1-50) | 0.464 |
| Yes | 4.4 ± 5.5 | 1 (0.2-13) | |
| Trauma | |||
| No | 10.4 ± 12.4 | 6 (0.1-50) | < 0.001 |
| Yes | 0.2 ± 0.1 | 0.1 (0.1-0.3) | |
| Lymphoedema | |||
| No | 9 ± 12.5 | 3 (0.1-50) | 0.015 |
| Yes | 15.7 ± 8.4 | 14 (8-31) | |
| Neuropathic leprosy ulcers | |||
| No | 9.4 ± 12.6 | 5 (0.1-50) | 0.174 |
| Yes | 12 ± 10.1 | 9 (1-30) | |
| Comorbidity | Mean ± SD | Median (minimum-maximum) | p* |
| Leprosy (current or previous) | |||
| No | 9.3 ± 12.7 | 5 (0.1-50) | 0.114 |
| Yes | 12.1 ± 9.4 | 10 (1-30) | |
| DM | |||
| No | 12.5 ± 13.5 | 7,5 (0.1-50) | 0.006 |
| Yes | 5.1 ± 8 | 1,5 (0.1-35) | |
| SAH | |||
| No | 10.8 ± 11 | 6,5 (0.1-33) | 0.378 |
| Yes | 9.3 ± 12.8 | 4 (0.1-50) | |
| Dyslipidaemia | |||
| No | 10.1 ± 12 | 6 (0.1-50) | 0.530 |
| Yes | 8.4 ± 13.5 | 5 (0.1-50) | |
| Other | |||
| No | 11.4 ± 12.6 | 8,5 (0.1-50) | 0.278 |
| Yes | 8.4 ± 11.9 | 4 (0.1-50) | |
*p-values obtained from Mann-Whitney nonparametric test. CLEU: chronic lower extremity ulcers; DM: diabetes mellitus; SAH: systemic arterial hypertension; SD: standard deviation.
Comorbidities found in CLEU patients are described in table 4, and medications used are described in table 5.
Table 4 Comorbidities in patients with CLEU
| Comorbidity | n (%) |
|---|---|
| SAH | 49 (72.06) |
| DM | 24 (35.29) |
| Dyslipidaemia | 15 (22.06) |
| Hypothyroidism | 10 (14.71) |
| Leprosy (current or previous) | 9 (13.24) |
| Cardiovascular disease | 11 (16.18)* |
| Cardiac insufficiency | 4 (5.88) |
| Atrial fibrillation | 4 (5.88) |
| Previous AMI | 3 (4.41) |
| Previous stroke | 3 (4.41) |
| Rheumatic cardiopathy | 1 (1.47) |
| Congenital heart disease | 1 (1.47) |
| Peripheral artery disease | 1 (1.47) |
| Neuropsychiatric disorders | 7 (10.29)* |
| Depression | 2 (2.94) |
| Anxiety | 3 (4.41) |
| Schizophrenia | 1 (1.47) |
| Epilepsy | 1 (1.47) |
| Cognitive impairment | 1 (1.47) |
| Musculoskeletal/osteoarticular disorders | 5 (7.35)* |
| Fibromyalgia | 1 (1.47) |
| Gout | 1 (1.47) |
| Osteoarthritis | 3 (4.41) |
| Osteoporosis | 1 (1.47) |
| Osteopenia | 1 (1.47) |
| Anaemia | 4 (5.88) |
| BPH | 3 (4.41) |
| Previous malignancy | 3 (4.41)* |
| Leukaemia | 1 (1.47) |
| Kaposi sarcoma | 1 (1.47) |
| Hypopharyngeal cancer | 1 (1.47) |
| Other | 7 (10.29)* |
| COPD | 2 (2.94) |
| Psoriasis | 1 (1.47) |
| HIV infection | 1 (1.47) |
| CKD | 1 (1.47) |
| Gastric ulcer | 1 (1.47) |
*The number of patients in each group of comorbidities may not correspond to the exact sum of its lower lines, as some patients had more than one comorbidity of the same group. CLEU: chronic lower extremity ulcers; SAH: systemic arterial hypertension; DM: diabetes mellitus; AMI: acute myocardial infarction; BPH: benign prostatic hyperplasia; COPD: chronic obstructive pulmonary disease; HIV: human immunodeficiency virus; CKD: chronic kidney disease.
Table 5 Medications used by patients with CLEU
| Medication | n (%) |
|---|---|
| Antihypertensives | 46 (67.65)* |
| ARB | 22 (32.35) |
| ACE inhibitors | 20 (29.41) |
| Beta blockers | 13 (19.12) |
| Diuretics | 12 (17.65) |
| Amlodipine | 9 (13.24) |
| Other | 2 (2.94) |
| Hypoglycaemic agents | 24 (35.29)* |
| Insulin | 11 (16.18) |
| Metformin | 18 (26.47) |
| Other | 3 (4.41) |
| Anticoagulants and antiplatelets | 19 (27.94) |
| Neuropsychiatric drugs/opioids | 16 (23.53)* |
| Opioids | 7 (10.29) |
| Gabapentinoids | 6 (8.82) |
| Antidepressants | 5 (7.35) |
| Anticonvulsants | 3 (4.41) |
| Other | 3 (4.41) |
| Statins | 15 (22.06) |
| Levothyroxine | 10 (14.71) |
| NSAID and pain killers | 8 (11.76) |
| Diosmin | 8 (11.76) |
| Omeprazole | 8 (11.76) |
| Vitamin and mineral supplements | 7 (10.29) |
| Antiarrhythmics | 2 (2.94) |
| Others | 10 (14.71) |
*The number of patients in each group of medications may not correspond to the exact sum of its lower lines, as some patients used more than one medication of the same group. CLEU: chronic lower extremity ulcers; ARB: angiotensin receptor blockers; ACE: angiotensin-converting enzyme; NSAID: nonsteroidal anti-inflammatory drugs.
Discussion
In the present study, patients have shown a similar epidemiological profile to the one found in other publications on CLEU in Brazil. A study5 performed in 2014 assessed 41 patients with CLEU in the city of Bauru, state of São Paulo, and the mean age of patients was 61.78 years, analogous to the one found in this study (65.10 years), with a predominance of male patients (58.54%) that was also similar to the one in this study (58.82%)5. In studies performed in other countries17,19,20, there was a greater variation in the mean age of chronic ulcer patients: from 38 years in Malawi and 56.6 years in Togo, to 69.9 years in Germany – reflecting differences in life expectancy and patient profile in these populations. The majority of our patients were elderly: 72.05% were 60 years or older – a bigger rate than in other studies in Brazil, from 46.53% to 61%4,5,24.
In the study conducted in Bauru5, there was a greater proportion of obese patients than in the current study: 43.90% (against 33.82%), and there was no case of low BMI (whereas 4.41% of patients in this study were underweight). Studies evaluating specifically patients with pressure injuries21-23,27 found a higher proportion of patients with nutritional status deficiencies: 56.6% of 122 patients in the city of Aracaju, in the Brazilian state of Sergipe, were either underweight or obese21; and an analysis of 324 institutionalized elderly in the city of João Pessoa, in the Brazilian state of Paraíba, found that patients with a nutritional status impairment had a 3.021 odds ratio of presenting a pressure injury22. A multicentric study23 assessing 473 patients in seven different Brazilian state capitals found malnutrition to be one of the main risk factors for the development of pressure injuries (odds ratio of 10.46). In that study, 98% of patients with pressure injury stage 2 or greater had some degree of malnourishment23. In the same way, a literature review27 showed greater odds of pressure injuries in patients with BMI lower than 18.5 kg/m².
A minority of patients (27.94%) in our study presented BMI within the range defined by WHO as normal weight. Nevertheless, BMI is a limited method for analyzing nutritional status. It is recognized the role of validated nutritional assessment tools such as Mini Nutritional Assessment, Subjective Global Assessment, and Global Leadership Initiative on Malnutrition criteria as important in the screening of malnutrition12,15,23,28,29. The Mini Nutritional Assessment, for instance, seems to be the best predictor for the risk of developing pressure injuries30. Knowledge on a CLEU patient's nutritional status may allow interventions that will benefit wound healing, such as a hyperproteic diet and nutritional supplements with amino acids (arginine and glutamine), vitamins (A, C, D, and folic acid), and minerals12,14.
In the present study, the rate of alcoholism was 17.65%, and the rate of smoking was 48.53%. Comparatively, smoking and/or alcoholism were present in 19.8% of 101 patients with venous ulcers in the city of Natal, in the Brazilian state of Rio Grande do Norte, and in 21.4% of 70 patients in the city of Évora, in Portugal4. Only 8.2% out of 122 inpatients with pressure injuries in Aracaju had a story of smoking21. Smoking is related to worse healing outcomes, besides contributing to the pathogenesis of some lesions, such as arterial ulcers11,16.
With respect to the etiology of CLEU, the data shown are similar to other national publications, with venous ulcers predominance (57.35% of patients in the current study). In the city of Bauru, São Paulo, the most frequent etiology was venous (49.80% of patients), followed by neuropathic leprosy ulcers (29.30%)5. In the city of Juiz de Fora, in the Brazilian state of Minas Gerais, venous etiology was present in 79.0% of CLEU patients, and the second most common cause was hypertensive ulcers, present in 15.4%24. In Germany17, when considering only chronic ulcers of the leg, the main etiology was venous (51.3%), followed by mixed venous and arterial (12.9%), and by arterial-only ulcers (11.0%). In the same study, vasculitis was the cause in 4.5% of lesions, pyoderma gangrenosum in 2.8%, and lymphoedema was present in 1.7% of patients17. The share of patients with lymphoedema here presented (10.29%) is closer to the one found in a study of 113 CLEU patients in London, published in the year 200431. In such study, 77.5% of ulcers were associated to CVU, and 14.5% out of the total were associated to lymphoedema31. In a study with 125 inpatients with CLEU in Togo, the main etiologies were infections, responsible for 49.6% of lesions - in this population; just 16.8% of lesions were venous ulcers19. In the same way, infections caused 50% of chronic ulcers in 44 outpatients in Malawi20.
Therefore, etiologies of CLEU in the studied population seem to have more similarities to that of more developed countries, except for the higher proportion of neuropathic leprosy ulcers (present in 11.76% of our patients) and of lymphoedema. Even though Brazil is the second country in the number of leprosy cases32, there is still little knowledge concerning this disease among the general population, and late diagnosis is common, which may lead to incapacities, neuropathy, and ulcers25. Lymphoedema affects severely life quality and is normally associated with venous insufficiency and severe obesity. It is also frequently associated with recurrent skin and soft-tissue infections and may indicate decreased access to healthcare17,33.
Diabetic foot ulcers and arterial ulcers are the main etiologies to be associated to an increased mortality in CLEU patients6 and were present in 11.76% and 8.82% of evaluated patients, respectively. In the study, there were no CLEU of hypertensive etiology, due to pyoderma gangrenosum or to vasculitis.
Although etiologies of CLEU were proportionally comparable to European and North American countries, the same was not true when considering the duration of CLEU. Patients assessed in this study presented lesions for a mean time of 9.7 years – with one patient reaching a maximum of 50 years without healing. The longest-lasting wounds were mostly venous ulcers. The mean duration of CLEU was 40.8 months in Germany; and the median duration was 8 months in London, 94.2 months in the Brazilian city of Juiz de Fora, and 48 months in the Brazilian city of Bauru5,17,24,31.
Long-lasting ulcers may express unmet needs for these patients, and greatly impair quality of life. De Oliveira Torres et al.4 showed reduction in quality of live in CLEU patients to be considerably higher in Brazil, in a background of greater socioeconomic insecurity, than in Portugal4. Moreover, the CLEU itself contributes to curtail productivity and worsen socioeconomic conditions of patients, thus affecting the whole society. Estimated costs in the United States are 15 billion dollars/year for venous ulcers, and 9-13 billion dollars for diabetic foot ulcers1.
Several factors may add to a longer duration of CLEU in the studied population. In the Brazilian public health system, there are no guidelines helping to standardize or guide care of chronic ulcers. In 2002, an international consensus34 developed a systematic approach regarding four important factors in wound healing (TIME guidelines) – guiding debridement for remotion of devitalized tissue and wound bed preparation, infection and inflammation control, moisture imbalance, and epithelial edge advancement7,34. Bacterial colonization occurs in most chronic ulcers7, and biofilm develops in at least 60-90% of it, damaging healing due to continued inflammation even in the absence of typical local infection signs2,7. It is unclear to what extent biofilm treatment has been addressed in CLEU patients in Brazil.
Wound size was also bigger in the present study: the mean area was 80.0 cm², and the median area 30.0 cm². In 70.37% of cases, ulcers had areas larger than 5.0 cm². Mean ulcer size was 43.7 cm² in a study carried out in Germany in 2014, and median size was 4.0 cm² in London in 200417,31. Wachholz et al. described 58.5% of patients as having lesions smaller than 4.0 cm² in Bauru, and 90% of patients described by Frade et al. in Juiz de Fora had lesions with at least one axis bigger than 5.0 cm5,24.
Comorbidities in the present study had a similar proportion to those found in other studies, and SAH and DM were the most frequent diseases found in patients with CLEU17,19,24,31. Among several assessed comorbidities, Matos et al. found occurrence of pressure injuries to be possibly associated only to neurological diseases and visual impairment22.
Limitations of this study include the fact that, as a cross-sectional study, it could not evaluate which factors could be associated to better outcomes in wound healings. Absence of a comparative control group and the small sample size may have prevented finding more statistical associations.
Conclusion
Although the clinical and epidemiological profile of CLEU patients in this study is comparable to countries in Europe and North America, there is a much larger proportion of CLEU caused by leprosy neuropathy, and of patients with lymphoedema. Duration and mean size of ulcers were larger than described in medical literature – what may indicate there is still much to improve in the care of CLEU patients in Brazil's public health system. Awareness about the influence of nutritional status on healing is important, as well as strict public health actions on smoking cessation.
Measures contributing to improved healing in these patients could benefit the entire society. CLEU is an important cause of reduced quality of life and of great morbidity and mortality, besides substantial socioeconomic costs. Even though, there are few studies about epidemiology of CLEU in developing countries such as Brazil. These studies could allow a better knowledge of the affected population, leading to better guidance on strategies for prevention and treatment. Longitudinal studies assessing factors that influence chronification of CLEU – such as nutritional status and adequate biofilm treatment – are also necessary.