Introduction
Epidermolysis Bullosa (EB) represents a group of rare congenital disorders caused by genetic variants in skin structural proteins, resulting in disruption of the dermal-epidermal junction. It is a clinically and genetically heterogeneous disorder characterized by skin fragility, with a propensity to impaired wound healing and blister formation. Based upon the ultrastructural levels of skin cleavage, EB is classified into four major types: simplex, junctional, dystrophic, and Kindler Syndrome. Recessive dystrophic EB (RDEB) results from a mutation in the COL7A1 gene that encodes the alpha-1 chain of type VII collagen1,2.
Squamous cell carcinoma (SCC) is one of the most feared complications and the leading cause of death of several subtypes of EB. The risk is higher for RDEB, especially in the generalized severe form3. To date, surgery is the first-line treatment for EB-associated SCC (EB-SCC), however, these tumors show an aggressive course, with high rates of recurrence even following complete excision4,5. Systemic chemotherapy and radiotherapy are only recommended as palliative modalities since their side effects may overweight the benefits in these fragile patients6,7. Despite all treatments available, there is a high morbidity and mortality associated to EB-SCCs, with significantly reduced life expectancy.
Electrochemotherapy (ECT) is a local treatment for cutaneous and subcutaneous tumors. The first ECT clinical trial was performed in 1991 in Villejuif, France, on head and neck cutaneous metastatic SCCs8. The European Standard Operating Procedures of ECT (ESOPE) project, which established the guidelines for a safe application of ECT in clinical practice was published in 20069. Even though ECT is mainly applied as palliative care for metastases, it may also be used for primary tumors that are unresectable due to size and location10,11. ECT has been proposed as a new treatment modality for EB-SCCs, due to its effectiveness and favorable safety profile4,12,13.
Case Report
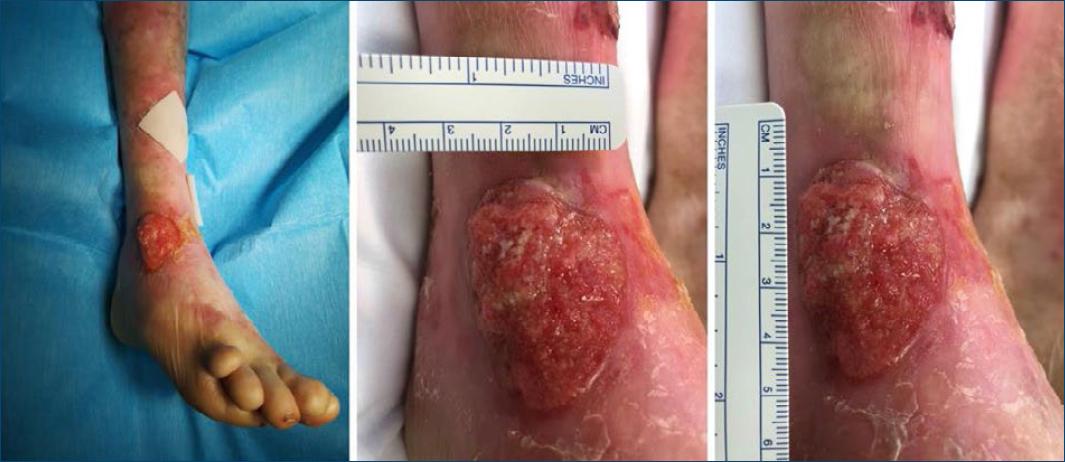
We report the case of a 38-year-old female patient with RDEB who presented with a cutaneous SCC in the dorsum of the right foot (Fig. 1). The lesion had been growing for the past 4 months, and was successfully treated with ECT, obviating the need for further treatments. The inclusion criteria and technical procedures followed the ESOPE9. Patient underwent an ECT session using the ePORE Gx device (Pes Med, Italy) in which bleomycin was administrated intravenously at a dose of 15 000 UI/m2 for 2 minutes, followed by electric pulses 8 minutes later. The lesion was examined and measured at follow-up appointments to evaluate the response to treatment and efficacy was assessed according to the Response Evaluation Criteria in Solid Tumours14. We reported stable disease 8 weeks after the first treatment, so we proposed a second session, which the patient consented. The second ECT session was performed 9 weeks after the first one, using the Cliniporator 1 device (IGEA, Italy). At 8 weeks of follow-up, we reported a partial response (Fig. 2a), with a complete response and skin healing of the treated area at 24 weeks of follow-up (Fig. 2b). ECT was well tolerated, with only mild adverse effects reported, which included muscle contractions during each pulse and local pain, erythema, and ulceration in the follow-up period. After the first three postoperative months, in which the patient was evaluated every two weeks, a quarterly follow-up was performed in the first year and every four months thereafter. At 64 weeks of follow-up, the patient is still free of recurrence.
Discussion
A major complication of EB is the development of multiple cutaneous SCCs, which generally develop in early adulthood and tend to arise at sites of chronic non-healing wounds or hyperkeratotic lesions. These tumors may be difficult to identify clinically since they frequently resemble areas of nonmalignant EB ulceration and wounds, so it is essential to maintain a straight clinical follow-up and have a low suspicion threshold to perform biopsy3,15. They represent the leading cause of death in EB at or after mid-adolescence, with death from cutaneous SCC occurring within 5 years of the diagnosis of the first SCC in most patients. The cumulative risk of at least one SCC in patients with generalized severe form of RDEB is 7.5%, 68%, and 90% by ages 30, 35, and 55 respectively. In contrast, the risk of these tumors is <25% by age 45 years in other forms of RDEB16.
The first line treatment for cutaneous EB-SCCs is surgery however these lesions tend to recur locally as their borders are usually indistinct4. Radiotherapy and conventional chemotherapy have been reserved as palliative modalities for advanced or locally recurrent SCC so other treatment options should be considered6,7. ECT combines the administration of a low dose of a chemotherapeutic agent, such as cisplatin or bleomycin, and the local application of short intense electric pulses. The electric fields transiently permeabilize the cell membrane, allowing the entrance of the drug into the neoplastic cells, enhancing its effectiveness and reducing its side effects. Only transient and mild adverse effects are usually reported8,10, as in the present case. Although it has no impact in the systemic progression of the disease, it has been proposed as a treatment modality for EB-SCC with high overall response.
In this case, we verified a complete response of a cutaneous SCC after two well tolerated sessions of ECT. This report aims to reinforce the potential of ECT as a viable approach for SCCs in patient with EB, without the high risk of functional impairment after surgical excision and no contraindications related to the disease.