The present case report emphasizes the importance of considering fungal etiology in immunosuppressed patients, in view of the morbidity and mortality of these clinical conditions. In addition, cultural findings identified are rare, highlighting the dimorphic character of Exophiala and the need for adequate care with the culture media and environment for the etiological diagnosis of these infections.
Introduction
Phaeohyphomycosis is a group of infectious diseases caused by filamentous or darkly pigmented fungi. They are named dematiaceous or melanized due to the deposition of melanin in their cell walls1-4. We often find these fungi in nature, soil, wood, and decomposing plant debris in subtropical and tropical climates2,5-7.
Infections caused by filamentous dematiaceous fungi are rare in humans and often related to immunosuppression, such as in neoplasia, HIV infection, and organ transplanted patients. According to ‘Transplant Associated Infection Surveillance Network’, the prevalence of phaeohyphomycosis in transplant patients is estimated at 2.7%8. However, it can also occur in immunocompetent hosts2,5-7.
Phaeohyphomycosis usually develops through traumatic inoculation of the skin and subcutaneous tissue with contaminated matter, with most reports occurring on rural and outdoor workers2,6. The infection manifests in four clinical forms: superficial, subcutaneous, invasive (affecting brain, central nervous system, peritoneum, bones, and/or lungs), and systemic (fungemia)2,4,9. The subcutaneous form is the most common presentation. It usually occurs as nodules or cutaneous abscesses (phaeohyphomycotic cysts), and verrucous, hyperkeratotic, or ulcerated plaques on the limbs, fingers, wrists, knees, and ankles2,4.
This study aims to report a case of cutaneous phaeohyphomycosis in an immunosuppressed patient. It also presents a concise literature review on this rare and significant fungal infection.
Case report
Male patient, 61 years old, from Parambu, Ceará, living in Cachoeirinha, Rio Grande do Sul, Brazil. The patient had a clinical history of hypertension, cardiopathy, and kidney transplant in 2016. He was medicated daily with oral prednisone 5 mg, tacrolimus 1 mg, pantoprazole 20 mg, and metoprolol tartrate 100 mg. He visited the Dermatology department due to a brownish lesion on the left inferior limb resulting from a laceration in a tile one year earlier.
Cutaneous examination revealed a well-defined and firm brownish hyperchromic nodule measuring 4 × 2 cm at the distal third of the left leg (Figs. 1a and b). A cutaneous punch biopsy was performed and material was sent to histopathological, mycological, and bacteriological examinations. Histopathology showed epidermal necrosis, a granulomatous reaction with histiocytes and giant cells in the deep dermis and hypodermis associated with a suppurative inflammatory infiltrate when stained by haematoxylin and eosin (H&E) (Figs. 2a and b). PAS (Fig. 3) and Grocott (Figs. 4a and b) stains revealed broad septate hyphae and yeast cells. There were no bacterial colonies after 48 h of incubation in culture and, in addition, the mycological analysis did not present fungal structures either.
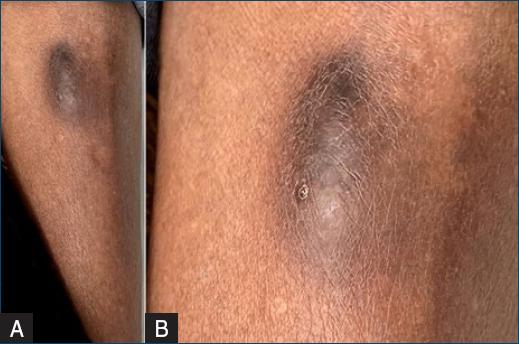
Figure 1 A: brownish nodule on the distal third of the left leg. B: with a detail showing a small crust.
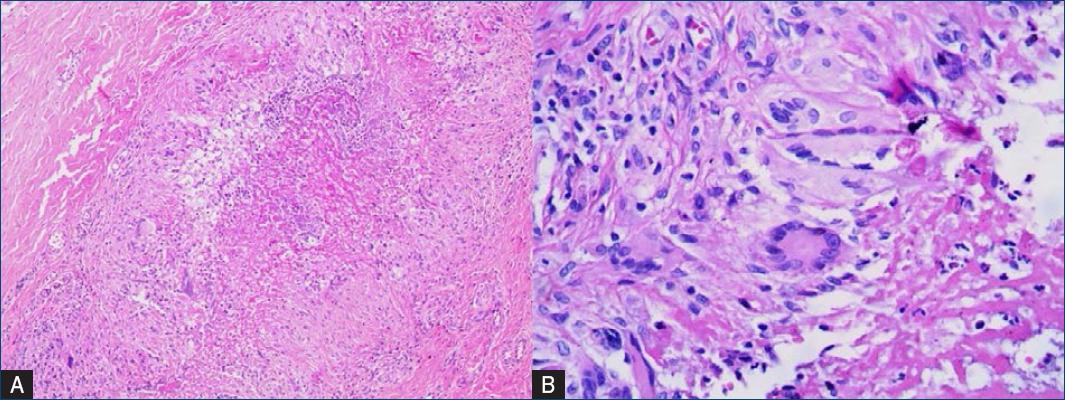
Figure 2 A: granulomatous reaction with histiocytes and giant cells, suppurative inflammatory infiltrate (H&E, ×20). B: a detail of lymphocytic infiltrate with multinucleated giant cell (H&E, ×40).
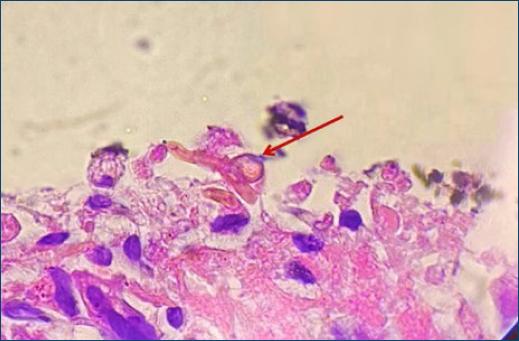
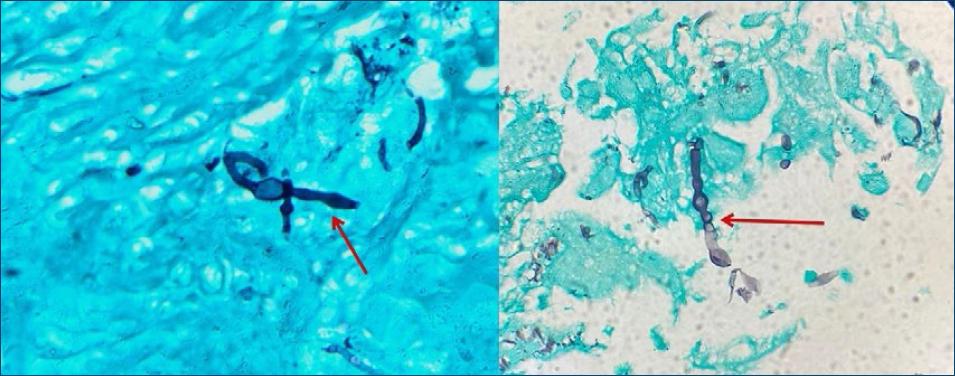
Figure 3 Presence of brownish hyphae and yeast cells in skin biopsy with lymphocytic infiltrate and histiocytes (PAS, ×100).
The culture in Sabouraud dextrose agar with chloramphenicol at 25°C showed an initial growth of a white filamentous velvety colony, that changed into a brownish coloration (Fig. 5a), with the presence of dematiaceous hyphae in microscopic examination (Fig. 5b). A smear was isolated and was analyzed through Polymerase Chain Reaction (PCR) and genetic sequencing, which identified the fungal species Exophiala dermatidis.
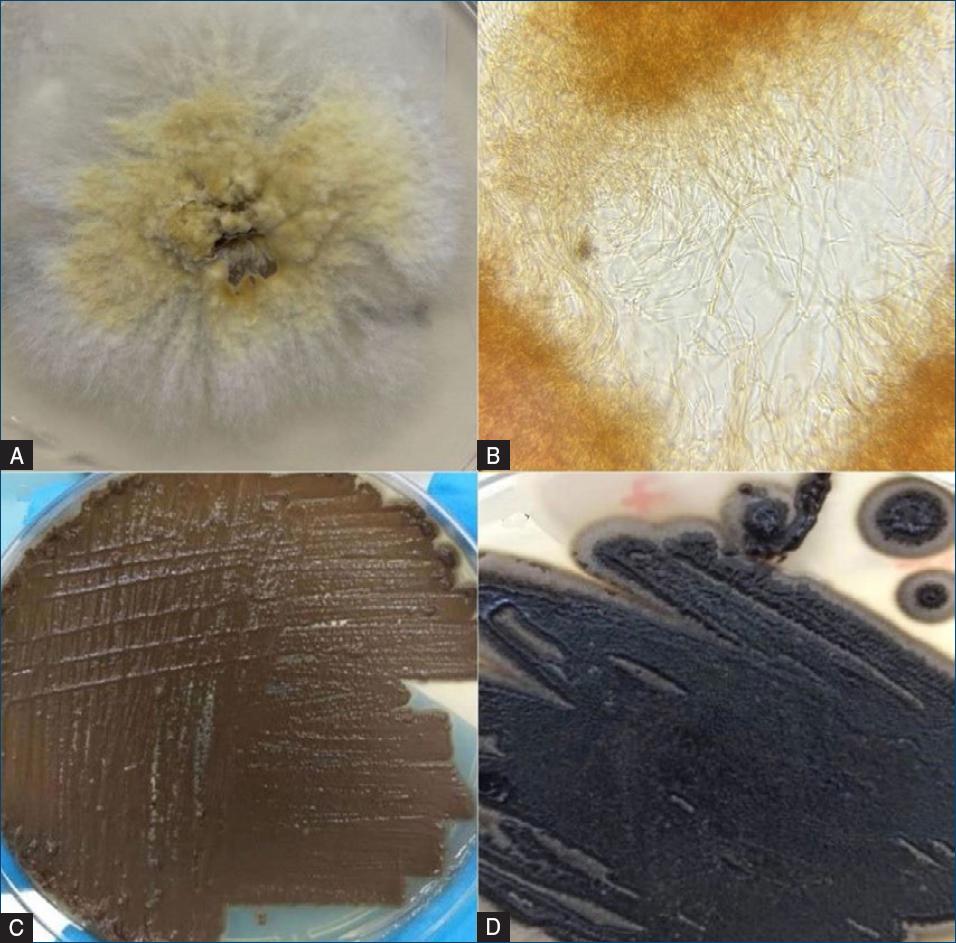
Figure 5 A: germination in Sabouraud dextrose agar with cloramphenicol at 25 °C: macroscopic aspect of the colony—front with a filamentous white colony that later received a brownish coloration. B: microscopy showing septate and ramified brownish hyphae, with no conidium. C: germination in potato agar at 35 °C showing creamy, yeast-like growth. D: followed by black yeast in filamentation process in potato agar at 25 °C.
Subsequently, to confirm the result of the fungal culture, a new sample was prepared in potato agar, stored at 35°C, revealing growth of a colony with black yeast-like aspect in 5 days (Fig. 5c). This isolate was identified by Matrix-assisted Laser Desorption Ionization-Time-of-Flight Mass Spectrometry (MALDI-TOF MS), confirming the species Exophiala dermatidis, obtaining a log-score value ≥2.0. Nevertheless, the plate was stored at 25°C for 10 days, and surprisingly, the agent restarted a filamentation process, demonstrating a colony of black filamentous morphology (Fig. 5d).
Ultrasound in the soft parts of the left leg identified a hypoechoic and heterogeneous ill-defined image in the distal third of the pretibial region. A computed tomography scan of the chest showed a focal/nodular pulmonary opacity with attenuation in ground glass at the posterior apical segment of the left upper lobe, measuring 2.4 cm and with a solid component measuring 0.4 cm, suggesting an inflammatory process and a primary proliferative lesion. We could not conclude our investigation due to the patient's death by Covid-19.
Discussion
Etiology and epidemiology
Dematiaceous fungi are widely distributed in nature and have the potential to infect humans. Dematiaceous fungi infections are classified into three groups: eumycotic mycetoma, characterized by the discharge of black grains via skin fistulae; chromoblastomycosis, characterized by infections with the presence of muriform cells in the skin and/or subcutaneous tissue; and the phaeohyphomycosis, in the form of septate dark hyphae, pseudohyphae, and yeast in the infected tissues1,6,10.
These fungi are part of the Phaeohyphomycetes class, whose main characteristic is the presence of dihydroxynaphthalene melanin pigment in the cell walls forming a brown pigment10,11.
The term phaeohyphomycosis was first introduced by Libero Ajello et al., in 1974. It comes from the Greek word phaios (dark), which refers to the brownish-black color morphology, septate hyphal, or mycelium filaments of fungi3,4,6,12. The most common genera are Exophiala sp., Wangiella sp., and Phialophora sp.2,6. Infection by Exophiala dermatitidis identified in our case, occurs through direct traumatic inoculation and/or wounds contaminated by the earth, decomposing vegetables, plants or wood2,13.
Due to its broad presence in nature, soil, and decomposing plant debris, subcutaneous phaeohyphomycosis fungi often affect rural populations, especially when the patient has a previous history of skin trauma. Infections often occur in tropical and subtropical climates, mainly in Central and South America.
Most infections develop in older adults with some degree of immunosuppression, usually associated with tuberculosis, diabetes, HIV infection or acquired immunodeficiency syndrome (AIDS), endocrine diseases, hematological malignancies, or other neoplasia, and also in transplanted patients and/or those under immunosuppressive therapies2,5,6, but the infection may also affect immunocompetent hosts2,7.
Phaeohyphomycosis is a rare infection, but the number of cases is growing, together with the number of immunocompromised patients with neoplasms and transplants2. In Rio Grande do Sul, the Brazilian state where we conducted this study, the first three cases of subcutaneous phaeohyphomycosis were reported at the end of the 1980s14.
As in the present case, immunosuppressive therapy in solid organ-transplanted patients predisposes for infections by filamentous fungi, resulting in much more serious disease and resistance to antifungal treatment13,15.
In this case, the identified fungal species, Exophiala dermatitidis is widely distributed in the natural environment, principally in warm and humid areas in tropical climates. Although uncommon, E. dermatitidis can cause cutaneous, corneal, and respiratory infections, affecting particularly patients with bronchiectasis or immunosuppression16,17.
The dimorphic character of E. dermatitidis is related with its pathogenicity, representing a virulence factor and an indicator of biofilm formation due to the morphological switch that is part of such formation process. Biofilm hinders the clearance of infections, resulting in a chronic recurrent infection17. Furthermore, melanin is believed to confer a protective advantage by scavenging free radicals and hypochlorite produced by phagocytic cells, protecting these microorganisms from the immunologic response2,10,11. This may explain the pathogenic potential of these agents4.
Classification and clinical manifestations
Phaeohyphomycosis represents a heterogeneous group of fungal infections, primary and opportunistic2,4. The most common clinical form is subcutaneous phaeohyphomycosis —also known as phaeohyphomycotic cyst— which can present under a wide range of skin lesions, such as papulonodules, nodules, verrucous, hyperkeratotic plaques, cysts, abscesses, and/or ulcers. They typically occur on the extremities such as feet, ankles, legs, and knees, areas that are more exposed to trauma2,4,7. Disseminated nodules of the subcutaneous form usually start on the limbs and spread over the body surface18.
At first, well-defined brownish mobile nodules slowly and chronically evolves into an encapsulated fluctuant mass with a liquefied center, that spares the underlying plans (muscles and bones)1,4,5, as in this case report.
Diagnosis
The diagnosis of subcutaneous phaeohyphomycosis can be difficult because of its many clinical forms. It depends on clinical, histopathology, and mycologic findings1,6. In the differential diagnosis, we can consider suppurating granulomatous lesions and other fungal infections such as sporotrichosis, leishmaniasis, and chromoblastomycosis1,18. Cultural examinations require expertise from the mycology laboratory15. Direct microscopy using potassium hydroxide may present septate hyphae, ramified hyphae (short or long; regular or curled), as well as yeast elements, and pseudohyphae1,13.
Histopathological examination of surgical specimens, using H&E staining, may present torus pigmented hyphae and yeast in the dermis and subcutaneous tissue, not affecting the epidermis1. These pigmented structures are helpful in the differential diagnosis of phaeohyphomycosis. In chromoblastomycosis, the diagnosis is established by muriform cells with septations along one or two planes in different levels of the tissue. In eumycotic mycetoma, fungi appear as microcolonies of hyphae forming grains13. Fontana-Masson staining, which highlights melanin pigment in cell walls, is useful to identify phaeohyphomycosis structures that are not seen in H&E1,4,6. However, it was not available in this study.
Histopathology determines the general diagnosis of phaeohyphomycosis but cannot identify the etiological agent, and after preservation of the material in formaldehyde fungus culture is no more viable13. In Sabouraud dextrose agar culture media, phaeohyphomycosis colonies are olive-green, brown, or black yeast like11. However, in this case, the Exophiala sp. presented initially as white to dark colonies with a velvety texture in lower temperatures, between 25-30 °C6. Therefore, temperature is an important factor regulating microbial activity and shaping this agent, due to the thermal dimorphism of some fungi.
PCR is a helpful technique for genetic sequencing of infectious pathogens, confirming the fungal species6,11. MALDI-TOF MS (Matrix-assisted laser desorption ionization time-of-flight mass spectrometry) consists of a fast, cheap, and reliable technique widely applied for fungal identification, mainly to yeast fungi19,20. In view of the dimorphism of fungal colonies, observed in this case report, samples were analyzed by both techniques with both identifying Exophiala dermatitidis. Despite the high diagnostic accuracy of such techniques they have limited availability and complexity and are more indicated to research studies6,11,19,20.
Treatment
Surgical resection is the treatment of choice for subcutaneous phaeohyphomycosis and results in cure and the absence of relapse in most cases. Surgery may adopt a conventional approach or use Mohs micrographic surgery, an efficient and conservative surgical treatment that minimizes tissue loss21.
Systemic antifungal therapy is recommended in relapse cases and immunocompromised patients. Therapy tends to have a long duration but there is no consensus on the length of therapy (6 weeks to 24 months6 ) and the choice of antifungals3. Itraconazole, voriconazole, amphotericin B, micafungin, colistin, terbinafine, and caspofungin have recommended even though they have demonstrated to be more effective in in vitro studies3,6,16,17. Isolated reports used terbinafine6. Itraconazole demonstrates activity against many dematiaceous fungi, but the medication is being less used due to side effects and the lack of an intravenous formulation3. Therefore, the literature lacks evidence that supports drug therapies for phaeohyphomycosis.
Conclusion
The wide range and little specificity of dermatologic manifestations complicate the clinical diagnosis of phaeohyphomycosis. However, considering the prevalence of dematiaceous fungi in the environment and the many pathogenic species, we must consider contamination even when agents are isolated by culture.
This report emphasizes the importance of cultural and anatomopathological additional examinations for the diagnosis of phaeohyphomycosis, especially in immunocompromised individuals, in which these infections are more frequent.
We also highlight the importance of proper analysis of macroscopic and microscopic morphology of fungal cultures, especially the singularities of each species, such as Exophiala dermatitidis, that demonstrated dimorphism in different temperatures. Analysis such as MALDI-TOF MS or gene sequencing may give a more accurate diagnosis, as in the present case.