Introduction
The attitude toward physical activity (PA) during pregnancy has changed over the last 50 years 1. In the past, when women became pregnant, were promptly advised to reduce or even discontinue the practice of PA due to the risk of complications for the mother and fetus 2. This was mainly due to sociocultural factors and a lack of scientific evidence to demonstrate safety in this practice 3.
Nowadays PA, defined as any voluntary bodily movement produced by musculoskeletal contraction that results in a substantial increase in caloric requirements over resting energy expenditure 4, is not only considered safe for the mother and fetus but also improves important pregnancy outcomes 5,6. Studies show that PA is closely associated with reducing the risk of obesity, hypertension, diabetes 7-9, cardiovascular disease 10,11, increased emotional well-being 12,13, reducing the risk of postpartum depression 7,8,14,15, preeclampsia 12, complications during childbirth 16, and risk of cesarean delivery 12.
There is a linear association between pregestational body mass index (BMI) and almost all adverse pregnancy outcomes 17,18. Pregnant women with an elevated BMI are at increased risk for the development of gestational diabetes mellitus, hypertensive disorders of pregnancy, and a need for unplanned cesarean deliveries. Their infants are also at increased risk for prematurity, stillbirth, macrosomia, birth trauma, respiratory distress, hypoglycemia, early postnatal infection, neonatal intensive care unit admission, and neonatal death 17.
Obesity in pregnant women, defined as pregestational BMI ≥30 kg/m2, is a significant public health challenge 19, worldwide, there are almost 15 million pregnant women with obesity 20. In the USA alone, 29% of women had obesity before becoming pregnant 19.
The proportion of pregnant women of reproductive age is increasing worldwide 21. In Portugal, 49.3% of the female population over 18 years old has excess weight or obesity 22, and a small study with 88 Portuguese pregnant women showed that 14.8% of them had obesity.
Pregnancy is a “window of opportunities” in terms of changing behavior and improving awareness of healthy living 23. There is increasing interest in research in the general population about whether reducing time spent in sedentary behaviors has a beneficial effect on health 24. Therefore, it became important to properly assess PA levels in different pregestational BMI pregnant women. PA evaluation determines trends, health benefits, and their effects over time 25. The purpose of this study was to (1) describe maternal PA levels and activities, stratified by pregestational BMI; (2) verify the accomplishment of PA recommendations in pregnant women with normative weight, pre-obesity, or obesity; (3) correlate pregestational BMI and PA accomplishment with maternal, delivery, and neonatal parameters.
Material and Methods
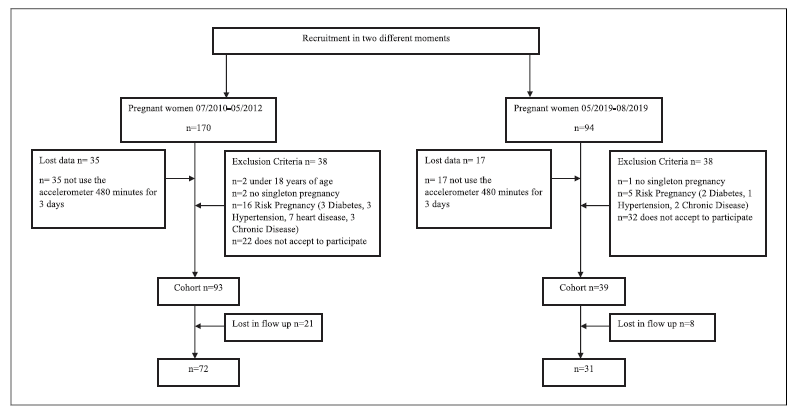
We conducted a retrospective cohort study at the University Hospital Center of São João (CHUSJ), Porto, at two different times. Women were recruited and assessed during medical appointments between July 2010 and May 2012, and May 2019 and August 2019. We exclude pregnant women: (1) those under 18 years of age; (2) diagnosed with insulin-dependent diabetes, hypertension, and/or cardiac diseases; (3) multiple pregnancies; and (4) those unable to follow or understand the procedure or who did not provide informed consent to participate in research 26,27. A total of 264 pregnant women were invited to participate in the study and 54 refused. Of those who agreed, 29 were lost in follow-up. The final sample, which included women who participated in both moments, consisted of 103 pregnant women. Figure 1 shows the flow chart of participants. We examined the following maternal outcomes: PA levels, gestational weight gain (GWG), excessive gestational weight gain (EGWG), and gestational diabetes mellitus (GDM); the delivery outcomes were: the type of birth and gestational age of birth; the newborn outcomes were: the Apgar score (1st and 5th min), weight at born, body composition, and head circumference. The sociodemographic data were collected by a standardized questionnaire.
Pregnant Women Evaluation
The pregnant women were recruited for our study during different gestational trimesters. PA evaluation was carried out once during the 1st, 2nd, or 3rd gestational trimester at the same time, we collected sociodemographic data. We followed up until delivery.
PA Measurement
Proper assessments of PA are important for determining both trends and health benefits and their effects over time. PA can be evaluated using objective methods, such as accelerometers and/or subjectively based on questionnaires 28. The PA was objectively assessed by accelerometry and subjectively assessed by questionnaire.
The accelerometers Actigraph GT3X® and wGT3X-BT® used in this study, monitored the acceleration of body segments and are considered a gold standard in objectivity and reliability (ICC:0.661-0.806) measuring sure PA levels 29. They have previously been used in pregnant women, including pregnant women with obesity, and showed good acceptability 26,30. Pregnant women wear the accelerometer on the waist for 7 consecutive days. Only those that record at least 480 miles daily, for a least 3 days were considered valid 29. The accelerometry of different intensity levels was accessed by Freedson 27 with cut points, which have already been used in other studies with pregnant women 31,32.
The Pregnancy Physical Activity Questionnaire (PPAQ) is a short-form questionnaire with 36 questions that aim to measure the type of activity and intensity of PA in pregnant women. It is a valid and reliable instrument (r = 0.09-0.43; ICC >0.78) that assesses the duration, frequency and intensity of household/caregiving, occupational, sports/exercise, transport and inactivity activities during the trimester and provides a quantitative measure of PA intensity which is classified as sedentary (<1.5 METs), light (1.5-3.0 METs), moderate (3.1-6.0 METs), or vigorous (>6.0 METs). One MET corresponds to the metabolic equivalent of energy expended at rest 32. It is a specific questionnaire for this population group and recommended in the literature to subjectively assess PA in pregnancy 33.
PA Recommendations
The American College of Sports Medicine (ACSM) recommends that pregnant women practice at least 150 min of moderate/vigorous physical activity intensity per week throughout the entire pregnancy 34.
Anthropometric Measures
The pregnant woman BMI’s was calculated using the Quetelet formula, which is based on individual height and weight - BMI = weight (kg)/(height)2 (m). The self-reported pregestational weight was confirmed in the Pregnant Health Bulletin, and the height was obtained through a fixed stadiometer (SECA 206). The weight was collected using a portable digital scale (Tantita InnerScan BC-545). According to the World Health Organization (WHO), the BMI can be categorized as underweight (BMI <18.4 kg/m2), normative weight (BMI 18.5-24.9 kg/m2), pre-obesity (BMI 25-29.9 kg/m2), and obesity (BMI ≥30 kg/m2) 35.
Gestational Weight Gain and Excessive Gestational Weight Gain
GWG was calculated as the difference between maternal weight at the last appointment (36-39 gestational weeks) and pregestational weight. The ideal GWG during pregnancy is defined by IOM (2009) and depends on pregestational BMI 36. The adequate GWG in normative pregestational BMI women ranges between 11.5 and 16 kg; in pre-obesity, between 7 and 11.5 kg and in women with obesity, it should not exceed 9 kg in the entire pregnancy. Values over these thresholds are considered EGWG 37.
Gestational Diabetes Mellitus
The presence of GDM was assessed between 24 and 28 gestation weeks through the oral glucose tolerance test. Blood sampling for fasting glucose concentrations was taken after a 10-h overnight fast, and glucose tolerance was measured by a 2-h 75 mg per-oral glucose tolerance test. GDM was diagnosed as fasting glucose ≥126 mg/dL or 2 h concentration ≥200 mg/dL 38.
Birth and Offspring Outcomes
The birth weight cut-offs were established by WHO Child Growth Standards as low birth weight (≤10th percentile), normal birth weight (>10th percentile <90th), and macrosomia (≥90th percentile) 39. For detailed analysis, the birth weight, length, and head circumferences Z-scores were predefined in the following percentiles: Z-score −4 to < −2 (0 percentile), Z-score −2 to < −1 (2nd percentile), Z-score −1 to < −0.5 (16th percentile), Z-score −0.5 to <0 (31st percentile), Z-score 0 (50th percentile), Z-score 0.5 to<1 (69th percentile), Z-score 1 to<2 (84th percentile), Z-score 3-4 (100th percentile). Data were collected by consulting the CHUSJ patient management computer system and Newborn Child Health Bulletin.
Statistics
Continuous data were presented in mean and standard deviation unless otherwise specified. Categorical data were presented as numbers and percentages. The F test in analysis of variance (ANOVA) was used to examine differences in characteristics between groups, for continuous variables. When normality was not assumed, we used the Kruskal-Wallis test. The χ2 test was used for categorical variables. The mother’s age, the newborn’s sex and the gestational week can influence maternal and offspring outcomes 40. These potentially confounding variables were adjusted to gestational week at birth in the multivariate logistic regression models to calculate maternal GWG, and offspring weight, length, and head circumference.
Statistical analyses were performed using SPSS for Windows, Version 18 (IBM, Armonk, NY). p < 0.05 was considered to indicate a statistically significant relationship.
Results
Sociodemographic Characteristics
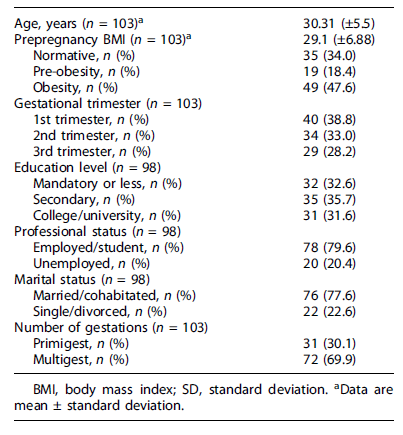
The mean age of women was 30.31 years (±5.5). Among 103 participants, 35 (34.0%) had a normative pre-pregnant BMI (range 20.8-24.5 kg/m2), 19 (18.4%) pre-obesity, and 49 (47.6%) obesity (range 30.4-47.5 kg/m2). At the recruitment moment, 40 pregnant women (38.8%) were in the first gestational trimester, 34 (33.0%) were in the second gestational trimester, and 29 (28.2%) were in the third. Thirty-one (31.6%) pregnant women had a high education level, 78 (79.6%) were employed, and 76 (77.6%) were married or cohabitated. The sociodemographic characteristics are shown in Table 1.
PA Assessed by Self-Reported Questionnaire
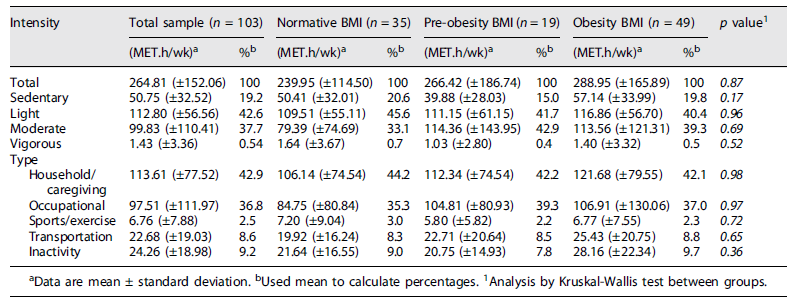
Table 2 represents maternal PA levels measured by PPAQ. The data show that pregnant women spent most of their time (42.9%) on household/caregiving activities and in occupational activities (36.8%). The results also show a very small percentage of energy expenditure in sports/exercise activities (2.5%). Regarding PA intensity measured by PPAQ, 42.6% of total activities were in light intensities, and just 0.54% of activities were spent in activities above 6METs. When the data were stratified by pregestational BMI, the results were similar between groups.
PA Assessed by Accelerometry
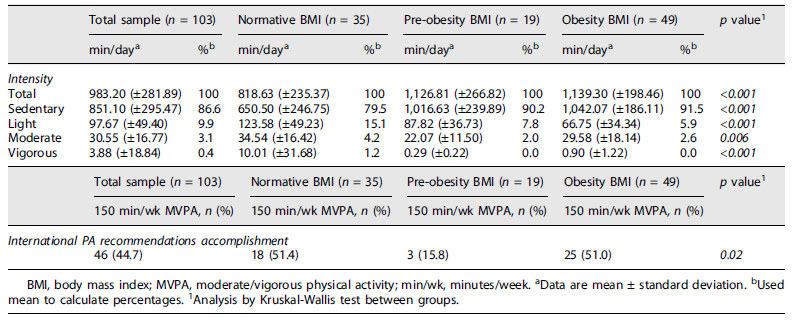
The accelerometry results (Table 3) indicate that pregnant women in the sample spent 96.5% of the time in activities below 3 METs of expended energy (light or below) and 3.1% of the time in moderate activities (between 3.1 and 6.0 METs). When data were stratified by pregestational BMI, we observed that women with normative BMI had higher levels in moderate activities, compared with pre-obesity and obesity BMI groups, with statistical differences between them (p = 0.006). Pregnant women with obesity spent 91.5% of their time in sedentary activities. Time spent in light-intensity activities in normative, pre-obesity, and obesity BMI groups were 15.1%, 7.8%, and 5.9%, respectively (p < 0.001). Regarding vigorous activities, pregnant women with normative pregestational BMI spent 1.2% of the time in activities over 6METs, and pregnant women with pre-obesity and obesity did not spend any time on activities of this intensity (p < 0.001).
International PA Recommendations
44.7% of participants reached the international recommendations for PA practice. In pregestational BMI groups, we found a statistically significant difference (p = 0.02). 51.4% cent of normative weight and 51.0% of women with obesity achieved the recommendations, while only 15.8% of pregnant women with pre-obesity reached the threshold.
Maternal, Delivery, and Neonatal Parameters
The results (Table 4) showed that pregnant women gained a mean of 13.09 kg (±6.41) during the entire pregnancy. Statistical differences were found (p = 0.03) between women who meet PA recommendations (11.21 kg (±6.21)) and women who did not meet the criteria (14.61 kg (±6.20)). Regarding pregestational BMI (Table 5), pregnant women with obesity gained lower weight (11.13 kg (±6.93)) compared to normative pregnant women (15.57 kg (±5.87)) with a statistical difference between groups (p = 0.01). However, the data showed that 68.4% of pre-obesity and 65.3% of obese pregnant women exceed the IOM recommendation for GWG.
Table 4 Relationship between maternal, delivery, and neonatal parameters to the international PA recommendations accomplishment group
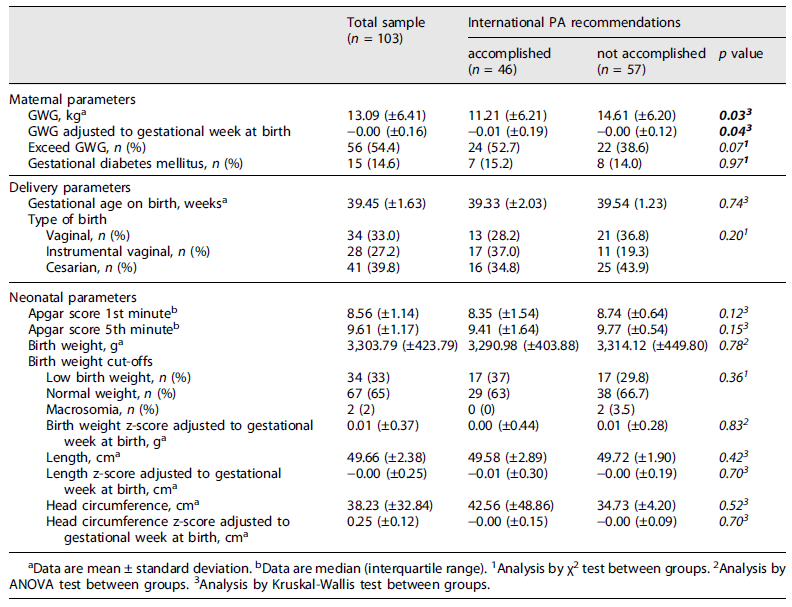
Table 5 Relationship between maternal, delivery, and neonatal parameters to the prepregnancy body mass index group
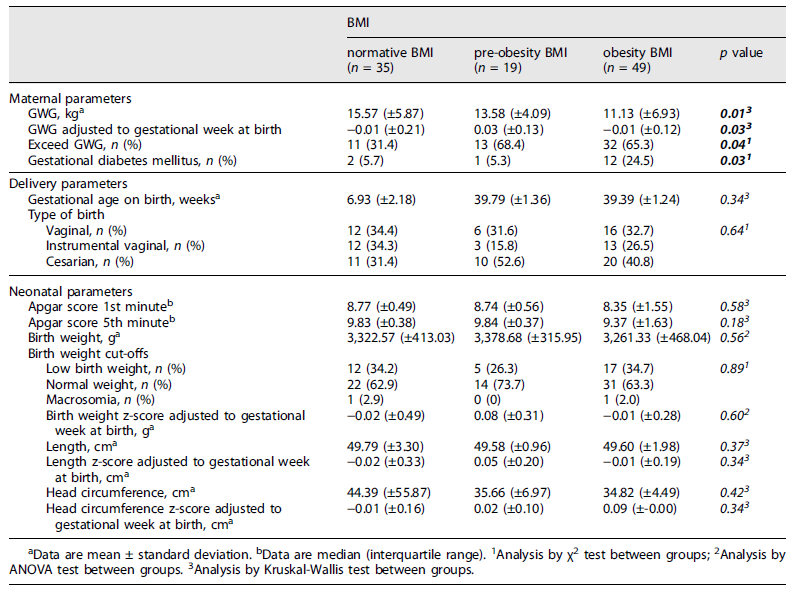
GDM was present in 14.6% of participants, with a statistical difference between pregestational BMI groups (p = 0.03). The obesity group had a GDM prevalence of 24.5% compared to 5.7% in the normative weight group.
39.4 weeks (±1.63) was the mean of the gestational age at birth for the total sample. 39.8% of total participants had a cesarean delivery, with the highest percentage in pregnant women who did not meet the international recommendations for PA practice (43.9%) and women with a pregestational BMI categorized as pre-obesity (52.6%). However, no statistical differences were found between the groups.
The neonatal parameters showed an Apgar score at the 1st and 5th minute of 9 and 10, respectively, the birth weight mean was 3303.79 g (±423.79), the length mean was 49.66 cm (±2.38) and the head circumference mean was 38.23 cm (±32.84). No statistical differences were found between those who met/not met PA practice recommendations or between pregestational BMI groups (p > 0.05). No statistical differences were found when values of potentially confounding variables (mother’s age, newborn sex) were adjusted.
Discussion
This study aimed to describe maternal PA levels and activities stratified by pregestational BMI, verify the achievement of PA recommendations, and correlate pregestational BMI and PA achievement with maternal, delivery, and neonatal parameters. Our key findings suggest that household/caregiving and occupational activities constituted the largest proportion of the total activity levels in pregnant women. Pregnant women with obesity had higher levels of sedentary behavior, higher prevalence of GDM, and exceeded the international recommendations for GWG. The prevalence of women who reached the recommended level of PA is 44.7%; however, only 15.8% of pregnant women with pre-obesity engaged in activities above the threshold.
In this study, we found that, independently of pregestational BMI, more than 79% of PA time was engaged in household/caregiving and occupational activities. These results support other authors who have suggested that domestic and obligatory activities (work-related) were those that contributed the most to energy costs and sports/exercise-related activities were not important contributors to energy expenditure during pregnancy (30, (41.
Regarding the PA intensity, measured by self-report questionnaire (PPAQ), pregnant women of the sample spent 19.2% of their time on sedentary activities, and we did not find differences between pregestational BMI groups. However, when objectively assessed by accelerometry, the results showed that pre-obesity and obesity groups spent more than 90% of their time in sedentary activities. In Chandonnet et al. 26 study, the explanation for the differences between assessment instruments might be that pregnant women with obesity spend more time in light-intensity activities, but perceive it as moderate or vigorous.
The findings of this study indicate that pregestational BMI was inversely correlated with PA levels. These results are in line with other works, a 2017 systematic review demonstrated that pregnant women spend at least half of their time in sedentary activities 24 and women with pre-obesity or obesity before gestation were at increased risk of sedentary behavior during pregnancy 42. Vigorous activities are extremely low in pregnant women 43 and women with obesity in general 44.
The regular practice of PA during pregnancy has shown multiple benefits. However notably, more than 50% of pregnant women in the sample failed to meet the recommendations of 150 weekly minutes of moderate/vigorous physical activity set in 2018 34. Nonetheless, results indicate that the rate of adherence to PA guidelines observed in this study is in line with Center for Disease Control (2018) 45 for the general population. When compared to other authors, we found that pregnant women in the sample had a higher rate of adherence to PA recommendations 46,47. This might be due to the fact that during pregnancy, women are in permanent contact with healthcare providers, and they encourage the regular practice of PA. Providers should be facilitators in promoting PA and lifestyle changes before and during pregnancy, especially in women with obesity who are typically more sedentary 48.
Pregnant women in the study gained 13.09 kg (±6.41) during pregnancy. The results have shown that women that did not achieve the PA recommendations gained more than 3.50 kg (mean) compared to those who meet the criteria. As occurred in other studies, PA and exercise seem to have a positive effect on GWG management 46,49,50. When analyzing pregestational BMI groups, we verified that although the normative weight group gained more weight during pregnancy, more than 60% of pregnant women in pre-obesity and obesity groups exceeded the IOM recommendations for GWG. These findings corroborate other studies that demonstrate that a high percentage of pregnant women with pre-obesity or obesity exceed the IOM recommendations for GWG 50-52. Healthcare providers to have a fundamental role in pregnant women’s health. They should encourage pregnant women to remain active during pregnancy and adopt adjusted and healthy diets.
Our study showed that there is a solid association between maternal obesity and GDM, which is in line with other studies 54-56. We did not find differences between groups that achieved or did not achieve PA recommendations. However, our results indicate that the normative weight group, which had higher levels of PA during pregnancy, was associated with a significantly lower risk of developing GDM, similar results were found in other studies 6-9,57-60.
We also observed that 33.0% of women in this study had a vaginal delivery, 27.2% instrumental vaginal, and 39.8% a cesarean section delivery. The data between groups have shown no statistical differences. However, a higher rate of cesarean section delivery was found in pre-obesity and obesity groups. The high rates of cesarean delivery were suggested to be due to a possible link between increased cholesterol deposits in the myometrium of women with obesity, affecting contractions, an increase in maternal soft tissue inside the pelvis narrowing the birth canal and increasing difficulty in births 54. Although our study has shown no statistical difference between pregnant who achieved and did not achieve PA recommendations, results from other studies support the benefits of PA, particularly in terms of reducing unplanned cesarean delivery 46,61,62.
Fetuses of obese gravidas are at an increased risk of macrosomia and impaired growth and preterm 17,55. Contrary to expectations, in our sample, we found no differences in birth weight cut-offs, length and head circumference, gestational age at birth and Apgar scores between pregestational BMI and achieve/not achieve PA recommendations groups.
Nevertheless, the findings of this study might present certain limitations. First, we only evaluate women in one gestational trimester. Due to that, we cannot verify PA changes during the entire pregnancy. Second, questionnaires are simple tools and easy to apply in research, however, self-reported measures can lead to possible classification errors 30. This concern was minimized through the use of a developed and validated questionnaire for the pregnancy population 28,32,63 and with the use of accelerometry which evaluates objectively the PA levels. Future studies with tailored PA and exercises programs, specifically in pregnant women with pre-obesity and obesity, are needed to explore the impact of these factors on the health and quality of life of mother and offspring.
Conclusion
Pregnant women with obesity, in this sample, exhibit high levels of sedentary behavior, a high prevalence of gestational diabetes, and exceed recommended GWG. Healthcare professionals have a crucial role in promoting regular PA and healthy lifestyle changes before and during pregnancy.
In the same way that nutrition programs for pregnant women have been effective, physical exercise programs conducted in person or at home-based, by healthcare providers could be a useful method for increasing PA during pregnancy. This is especially true for populations with comorbidities such as pregnant women with obesity.
Statement of Ethics
The Institutional Review Board of CHUSJ approved the original studies (n° 165/19) and reference number (09988). The use of the PPAQ questionnaire for this study was authorized by the author of the validation and adaptation for the Portuguese language and population. The study was conducted following the World Medical Association Helsinki Declaration for Human Studies. All participants provided written informed consent.
Conflict of Interest Statement
The authors also declare that have no conflicts of interest to declare.
Author Contributions
The conception, study design, and data acquisition were Diana Bernardo and Paula Clara Santos responsibility. Diana Bernardo and Carlos Carvalho analyzed and interpreted data and drafted the manuscript. The critical review regarding important intellectual content was carried out by Margarida Ferreira, Jorge Mota, and Paula Clara Santos. All authors approved the final version of the manuscript.