Dear Editor,
Patients with acute-on-chronic liver failure (ACLF) have high short-term mortality [1]. Over the past few decades, better access to intensive care and liver transplant (LT) has improved these patients’ outcomes [2]. However, concerns remain regarding futility of care, especially in patients with ACLF grade 3, the ones with the greatest severity of disease [3]. Not only their short-term survival following LT may be lower but they also frequently face several complications, namely, infection and different organ dysfunctions [4].
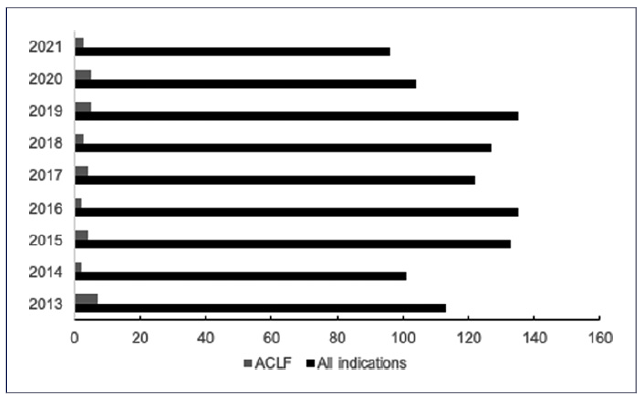
In the south LT region of Portugal, between 2013 and 2021, ACLF (defined as per the European Chronic Liver Failure Consortium) represented a median (interquartile range) of 3.1% (2.4-4.3%) of all indications for LT (Fig. 1: a maximum of 6.2% in 2013 and a minimum of 1.5% in 2016) [5]. Among a total of 186 patients with cirrhosis admitted for more than 24 h to the intensive care unit (ICU) at our center between 2013 and 2021, the ACLF grading on ICU day one was as follows: grade 0 in 7 (3.8%) patients, grade 1 in 46 (24.7%), grade 2 in 55 (29.6%) patients, and grade 3 in 78 (41.9%). Median (interquartile range) SOFA score on ICU day one was 12 (10-14). Overall, 35 (18.8%) patients received an LT (31 within the index hospital stay and 4 following hospital discharge). LT was significantly associated with lower 1-year all-cause mortality (Fig. 2: Kaplan-Meier curve with Breslow test p =0.001). Moreover, patients transplanted during the index hospital stay (emergent LT) had significantly higher median hospital length-of-stay (48 vs. 21 days, p < 0.001). There was no association between year of enrollment and these outcomes.
Our 1-year posttransplant crude survival was lower than described elsewhere [6]. Several reasons may help explain such discrepancy, for example, (1) the relative number and severity of organ failures in ACLF 3 patients; (2) local evolving practices for selecting patients for transplant;(3) timely access to suitable organs in different regions; or (4) the quality of organs available across countries.
Nevertheless, our results add to the increasing evidence highlighting that patients with ACLF may derive a substantial survival benefit from LT. However, associated morbidity and costs need to be taken into account as well. Furthermore, ethical concerns remain regarding futility and the fair distribution of scarcely available organs among patients with different indications for LT. In the future, hospitals will probably be under pressure to admit more of these patients to higher levels of care, especially the ICU. Thus, the complex planning of systems to deliver high quality of care will need to include these patients. Furthermore, clinicians will likely be confronted with more difficult decisions regarding the selection of patients with ACLF for LT. Therefore, future studies will be needed to improve our ability to identify those patients with ACLF who have the highest chances of surviving the longest following LT.