Introduction
Pancreatic solid-pseudopapillary neoplasms (SPN) are rare, and 90% occur in women in their 3rd and 4th decades of life [1]. The symptoms are nonspecific or absent and are mostly incidentally diagnosed during imaging exams such as abdominal ultrasound, computerized tomography (CT), or magnetic resonance cholangiopancreatography imaging (MRI/MRCP) [2]. SPN appears as a circumscribed, encapsulated, solid mass but eventually with cystic areas [3]. Most patients have localized disease, and up to 15% have metastasis or vascular invasion [4]. Conventional treatment is surgical resection, and unlike pancreatic ductal adenocarcinoma (PDAC), the result is favorable with a 5-year survival rate of 97% [1].
Based on isolated imaging exams, the accurate diagnosis of SPN is difficult, especially in solid or solid/cystic le-sions, and can commonly mimic a nonfunctioning pancreatic neuroendocrine tumor (NF-NET), serous cystad enoma (SCA), and less commonly a PDAC [5]. Surgical indication is based on the presence of a pancreatic nodule, usually leading to the execution of a procedure with a high morbidity and mortality rates without a preoperative diagnosis and previous anatomopathological confirmation. Endosonography (EUS) is increasingly being used in the evaluation of pancreatic tumors. The classificatory diagnosis made by preoperative EUS-guided fine-needle aspiration (EUS-FNA) (NF-NET <2.0 cm or SCA) would prevent unnecessary surgery. Recently, some studies evaluated the role in EUS-FNA in SPN [6-11], as well as comparing it with CT [12] and MRI for diagnosis, but its accurate performance in SPN diagnosis remains unclear. The aim of this study was to describe the imaging findings (CT and MRI/MRCP) and compare them to the accuracy of the EUS-FNA in the preoperative differential diagnosis of pancreatic solid or solid/cystic lesions.
Materials and Methods
This was a retrospective and multicentric case series, carried out in three reference university hospitals and two private tertiary referral hospitals in Brazil. The study was approved by the Research Ethics Committee of each institution and complied with the regulations of the Health Insurance Portability and Accountability Act (HI-PAA). The study was approved by the Research Ethics Committee on 07/22/2019 under number: 3.518.999.
The medical records of all patients submitted to EUS-FNA in the digestive endoscopy sectors of each of the centers involved in the study, between January 1997 and January 2020, were retrospectively reviewed. The selected patients had EUS-FNA before surgery and had suspected diagnosis of SPN, NF-NET, SCA, or other type of pancreatic lesion at CT, MRI/MRCP, and EUS with postoperative pathological evaluation detected SPN. All selected patients signed an informed consent form before undergoing EUS-FNA. Age, sex, symptoms and CT, MRI, and EUS-FNA findings were noted, and the final diagnosis was obtained after the histological examination of the surgical specimen in all patients.
EUS was performed with conscious sedation or anesthesia monitored by an anesthesiologist. All procedures were performed by experienced doctors, always using a linear echoendoscope. All biopsies were made under the same procedure. Specifics of the fine-needle gauge (19 G, 22 G, 25 G, and 20 G), number of needle passes, and results of microhistology (McH), in addition to the surgical pathology results, were recorded. The occurrence of adverse events (AE) was also documented.
Sample Handling
Samples obtained by FNA were deposited in 10% formalin (6-24 h) and followed the routine of the pathological anatomy sector of each center participating in the study. The blocks and biopsies embedded in paraffin were subjected to semi-series histological sections of 3-μm thick, at three different depth levels, stained by the hematoxylineosin (H/E) method. CellSens Micro Imaging Software (Olympus America Inc., Center Valley, PA, USA) was used to dimension samples in millimeters.
The diagnosis of SPN was confirmed through histology with H/E and, when possible, immunohistochemistry (IHC). The sec-tions stained with H/E from both FNA and surgical samples were reviewed by specialized pathologists to confirm the diagnosis. The IHC markers (primary antibodies) used in the analysis included β-catenin, CD10, progesterone receptor, CD99, and chromo-granin A [13].
Statistical Analysis
The collected and recorded data were stored in a database. The statistical analysis of continuous variables was described as mean and standard deviation and dichotomous variables expressed as simple proportions. The definitive diagnosis of SPN was based on surgical histological analysis specimen, and the patients who did not have it were excluded from the study. True-positive cases were the ones that after undergoing CT, MRI/MRCP, EUS, and McH had the SPN diagnosis histologically confirmed. True-negative cases were the ones that after undergoing imaging test or McH had the NF-NET or SCA diagnosis like a final diagnosis. False-positive cases were the ones that after undergoing imaging test or McH had the SPN, but the final diagnosis was different. False-negative were the ones that after undergoing imaging tests or McH revealed a different diagnosis from the SPN. To assess the diagnostic gain acquired with the combination of imaging tests, the numbers of tru-ly positive cases from each method were added. The comparison between the results obtained in the imaging exams was performed using the McNemmar test for each pair of groups (CT + EUS; CT + EUS-FNA; MRI + EUS; and MRI + EUS-FNA). The association between the size of the needle used and the diagnosis obtained in the McH was tested using Fisher’s exact test. The level of statistical significance adopted was 95%. The analyses were performed using the Stata 14 software.
Results
Fifty four patients with suspected SPN were initially studied. Three patients had a NF-NET tumor type G1 as a final diagnosis after surgical resection. The others (n = 51) had the diagnosis of SPN confirmed by McH, anatomopathological report of the surgical specimen, and IHC analysis. Of the 51 patients with confirmed SPN, 90.2% (n = 46) were female and 9.8% (n = 5) were male, and the mean age was 33.6 years (SD 14.46) (Table 1).
Table 1 Characteristics of patients submitted to EUS-FNA with suspected diagnosis of SPN on imaging exams in 5 different endoscopy centers in Brazil between January 1997 and January 2020
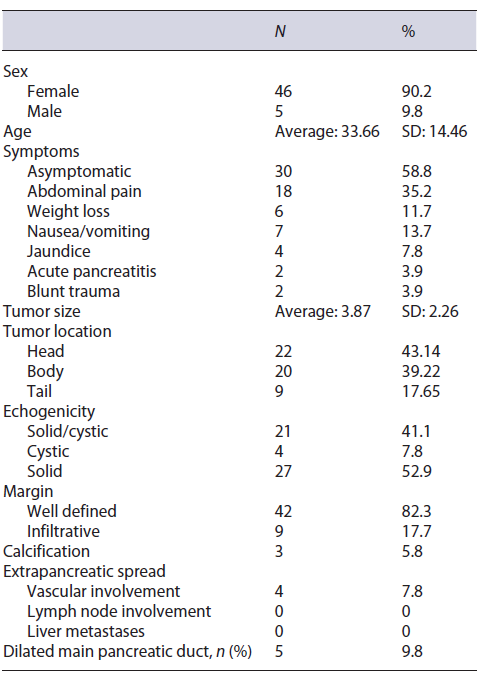
It was noted that 21 (41.2%) patients were symptom-atic, being the most common symptoms abdominal pain and weight loss associated with nausea or vomiting. Jaundice was present in 4 (7.8%) patients. SPN was detected incidentally in 32 (59.3%) of the patients. The average size of the lesion was 3.8 cm (SD 2.26), and the lesions were distributed between the body (20), head/uncinate (22), and tail (9) portions of the pancreas. One patient had two synchronous lesions identified on the body and tail of the pancreas. The solid, solid-cystic (microcystic), and cystic appearance was present in 27 (52.9%), 21 (41.1%), and 4 (7.8%) cases, respectively (Fig. 1). The lesion margin was well defined in 42 (82.3%) of the cases. Calcification was present in 3 (5.8%) patients. In 4 (7.8%) patients, there was vascular involvement detected by the EUS and confirmed by surgery, as well as dilation of the main pancre-atic duct in 5 (9.8%) patients (Table 1).
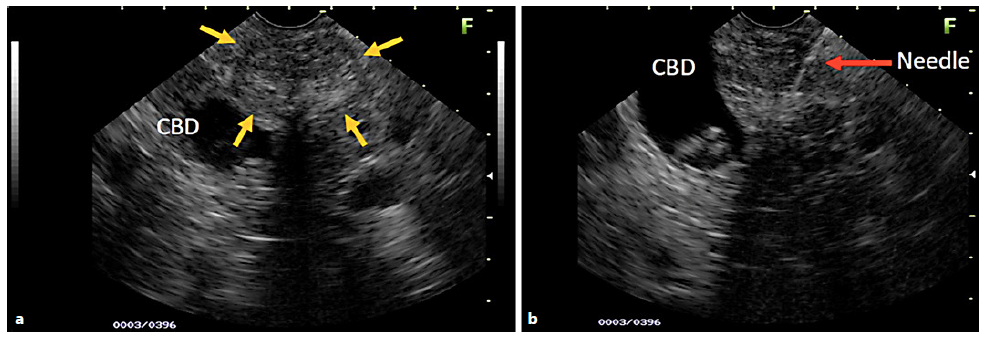
Fig. 1 a) EUS image of a young patient with a hypoechoic area (yellow arrows), also heterogeneous with imprecise limits and a microcystic component, right next to the common bile duct (CBD) with stones inside (a, b). The EUS images suspected SPN. B) Moment of the EUS-FNA (red arrow), with the 22-G needle positioned in the center of the lesion. The biopsy revealed chronic inflammatory process without evidence of SPN.
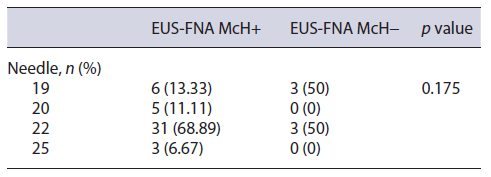
EUS-FNA was performed on all patients, without the pathologist’s presence of a rapid on-site evaluation (Table 2). The needles of 22 G in 34 (66.6%), 19 G in 9 (17.6%), 20 G in 5 (9.8%), and 25 G in 3 (5.8%) were used. A median of 2.5 needle passes (range 1-5) was performed for each lesion. The diagnostic performance of the EUS-FNA was not affected by the size of the needle (p = 0.175). No AE were documented in this case series. Surgical resec tion was performed in all patients who had no evidence of lymph nodes or distant metastases.
Table 2 Relationship between the gauge used to perform fine-needle aspiration and the final histopathological result in patients with suspected SPN from 5 different endoscopy centers in the city of São Paulo between January 1997 and January 2020
Preoperative Diagnostic Yield of CT, MRI, and EUS-FNA
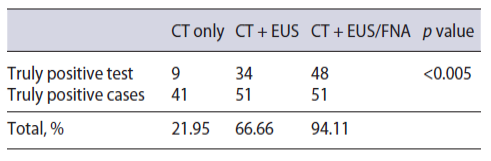
CT was performed in 44 patients. PDAC was suspected in 17 (38.67%), SPN in 10 (22.72%), pancreatic cystic lesion in 4 (9.09%), NF-NET in 4 (9.09%), chronic pancreatitis in 3 (6.81%), SCA in 2 (4.54%), pancreatic pseudocyst in 2 (4.54%), hematoma after blunt trauma in 1 (2.27%), and common bile duct dilation in 1 (2.27%) case (Fig. 2 ). The combination of CT + EUS and CT + EUS-FNA significantly increased (p < 0.005) the diagnosis compared to CT only, which went from 22.72% to 66.66% and 94.11%, respectively (Table 3). The sensitivity, specificity, positive and negative predictive value, and accuracy for the SPN diagnosis obtained by CT was 22%, 66.7%, 90%, 6%, and 25%, respectively.
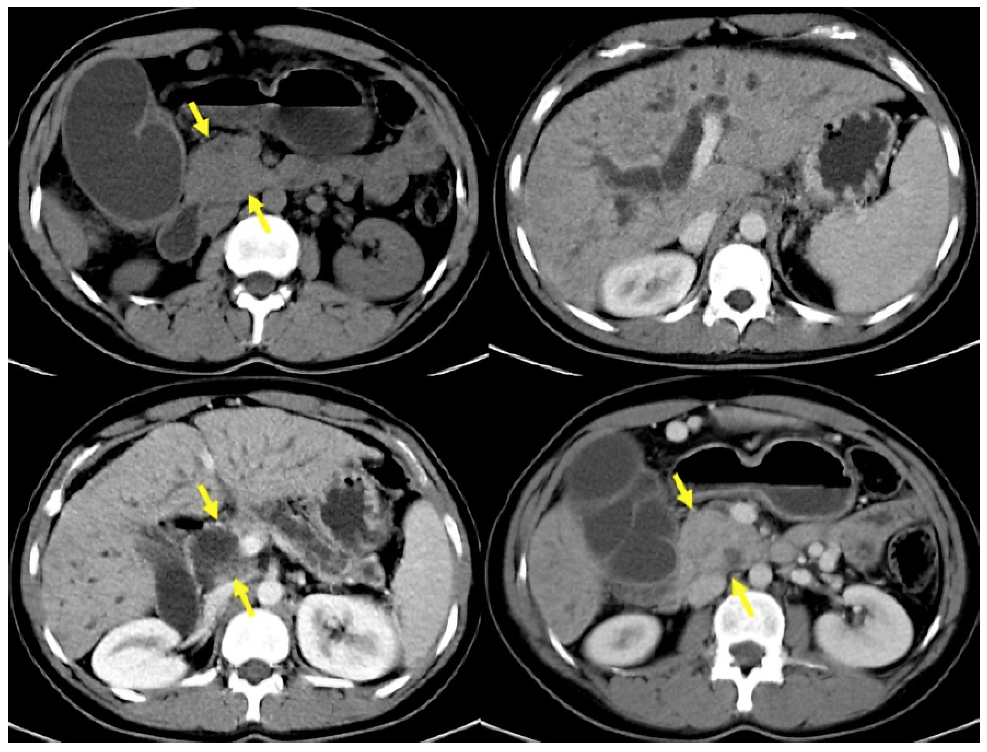
Fig. 2 CT images with enlarged pancreatic head, dilatation of the main pancreatic and choledochal duct, in addition to atrophy of the pancreatic gland in a 23-year-old woman with a history of chronic alcoholism (same case in Fig. 3).
Table 3 Performance of imaging exams, including CT, in relation to the total number of cases that were submitted to each group of imaging exams and that later proved to be truly positive through postsurgical histological diagnosis
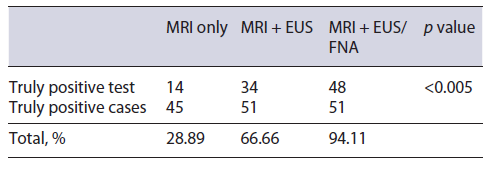
MRI/MRCP was performed on 48 patients. PDAC was suspected in 14 (29.16%), SPN in 14 (29.16%), pancreatic cystic lesion in 7 (14.56%), pancreatic pseudocyst in 4 (8.34%), NF-NET in 4 (8.34%), SCA in 2 (4.16%), chronic pancreatitis in 1 (2.08%), choledochal dilation in 1 (2.08%), and normal in 1 (2.08%) case (Fig. 3). The combination of MRI/MRCP + EUS and MRI/MRCP + EUS-FNA significantly increased (p < 0.005) the diagnosis compared to isolated MRI/MRCP, which went from 29.16% to 66.66% and 94.11%, respectively (Table 4). The sensitivity, specificity, positive and negative predictive value, and accuracy for the SPN diagnosis obtained by MRI was 31.2%, 66.7%, 93.3%, 6.1%, and 33.3%, respectively.
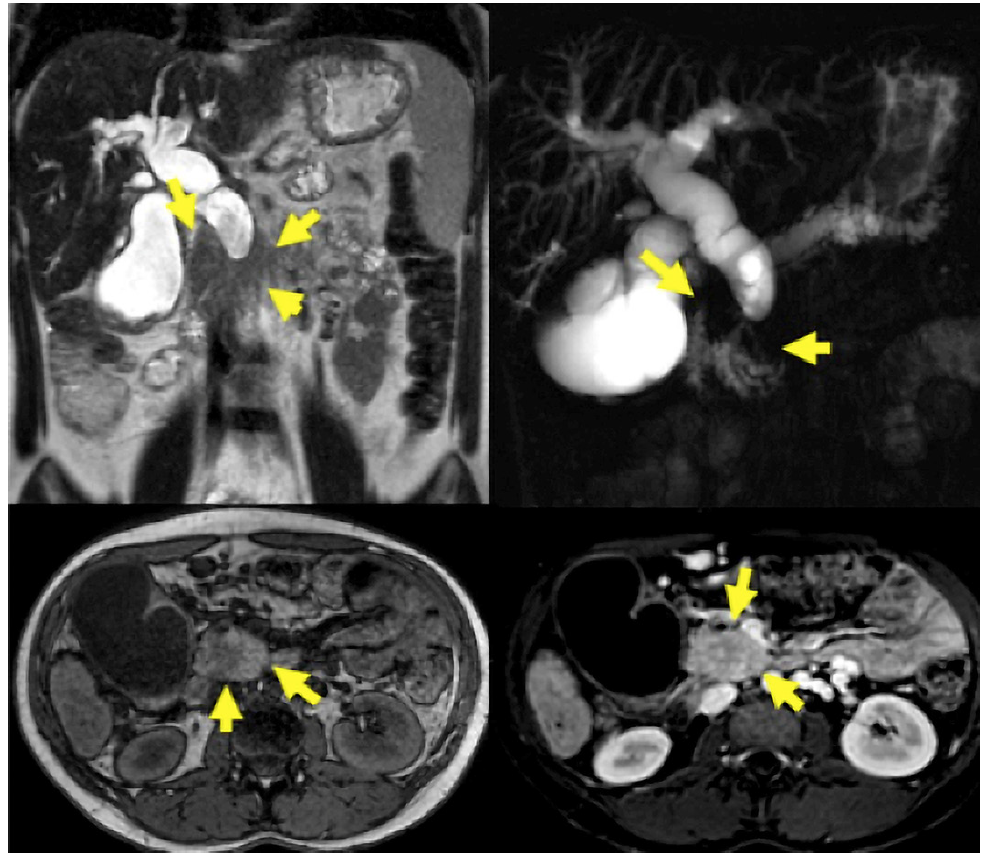
Fig. 3 A 23-year-old woman with signs of chronic pancreatitis on MRI/MRCP and an enlarged pancreatic head.
Table 4 Performance of imaging tests, including magnetic resonance imaging, in relation to the total number of cases that were submitted to each group of imaging exams and that later proved to be truly positive through postsurgical histological diagnosis
EUS alone suspected the diagnosis of SPN in 35 (64.85%). Two cases were not included in this analysis because they received a final diagnosis of NF-NET and had a previous result of SPN through EUS. EUS suspected PDAC in 9 (16.65%), NF-NET in 5 (9.25%), chronic pancreatitis in 1 (1.85%), pancreatic cystic lesion in 1 (1.85%), SCA in 2 (3.70%), and lymph node in 1 (1.85%).
The McH obtained by the EUS-FNA diagnosed SPN in 45 (88.24%), NF-NET in 2 (3.92%), and was negative in 4 cases (7.84%), all confirmed as SPN after surgery. The sensitivity, specificity, positive and negative predictive value, and accuracy for the SPN diagnosis obtained by isolated EUS and EUS-FNA was 68.6%, 33.3%, 94.6%, 5.9%, and 66.7% and 90.2%, 66.7%, 97.9%, 28.6%, and 88.9%, respectively.
Discussion/Conclusion
SPN are rare [14], and the ultrasound and CT are the most used imaging methods for diagnosis and represent approximately 80% of imaging studies for the diagnosis of SPN. In the past few decades, the use of MRI has increased substantially, as has EUS, but it still accounts for only 5% of the imaging methods used for the diagnosis of SPN. In addition, there are few studies that determine the additional benefit offered by the EUS-FNA to imaging tests such as CT and MRI [15]. The benefit of the use of EUS in patients with solid and cystic pancreatic tumors with a yield approaching 78.8% and 71.4%, respectively, has been proven [16, 17]. Therefore, the inclusion of EUS-FNA increases the diagnostic accuracy of this type of tumor.
The results of this study highlight the difficulty of CT, MRI, and EUS in correctly classifying the SPN using only the image resources, with a diagnostic performance of 21.95%, 28.88%, and 64.71%, respectively. These results are similar to another recent study, which was 23.5% and 41.2% for CT and EUS, respectively [12]. When we include the results of EUS-FNA, there is an increase in the diagnostic performance of CT (72.16%) and MRI (65.23%), rising up to 94.11% in both methods.
Classically, SPN are described as circumscribed, hypoechoic, solid, heterogeneous masses, and eventually with a cystic component. However, this appearance presents in just 60% of patients, with a predominantly solid lesion found in 32% of cases, suggesting a more classic appearance associated with an NF-NET [18]. In our series, the solid/cystic, solid, and cystic morphology was 52.9%, 41.1%, and 7.8%, respectively. SPN can mimic other pancreatic tumors, which can lead to diagnostic challenges [19]. This fact can be observed in our study, as CT and EUS suspected the presence of NF-NET in 9.75% and 7.8%, respectively, since the injuries identified by these exams had a predominantly solid component. When microcystic, SPN are similar to SCA and can cause diagnostic confusion, which occurred in 4% of the cases examined by CT, MRI, and EUS. The microcystic pattern must be recognized as part of the morphological spectrum of the SPN, which can lead to confusion regarding the presence of an SCA [5]. Previous CT and EUS studies, in which SPN was identified as other cystic lesions in the pancreas in up to 50% of cases, including benign lesions such as SCA, confirm the findings of our study [12, 20, 21].
Some cases were interpreted as pancreatic pseudocysts, highlighting the difficulty in determining the etiology of a cystic lesion based only on morphological characteristics of the image. This occurred in 7.31% and 15.55% of CTs and MRIs, respectively. Therefore, the importance of correctly classifying SPN is emphasized in this study, where 8 (33.3%) cysts were erroneously classi fied by CT, as pseudocysts (2), unspecific pancreatic cystic lesion (2), unspecific pancreatic cystic lesion with calcification (2), hematoma (1), and SCA (1), all which have prognosis and management completely different from those adopted in SPN. The same happened with MRI/MRCP which was normal in 3 patients and identified unspecific pancreatic cystic lesion in 4 and pseudocyst in 3 patients.
In the literature, rupture of the SPN is associated with abdominal trauma or may be spontaneous, which is rare.
To date, there have been only 3 cases of SPN with spontaneous rupture reported worldwide [22]. In our series, we had two young patients with blunt abdominal trauma who underwent imaging exams that suspected hematoma (1) and pancreatic pseudocyst (1). In these two cases, the EUS-FNA was crucial for the diagnosis of SPN, changing the management of these patients.
The ultimate aim of this study was to confirm the benefit of performing the EUS-FNA to increase the diagnostic performance of other conventional imaging tests such as CT and MRI. The EUS-FNA association increased diagnostic yield by almost 72.16% for CT and 65.23% for MRI. The results are similar to previous studies, which report a low sensitivity rate and absence of AE as in the current series [12]. Regarding the AE resulting from the EUS-FNA, the first case of neoplastic cell implantation in the stomach was recently described [23]. In our series, even after prolonged follow-up and interviews with patients and attending physicians, we did not observe this type of complication.
Two particular cases deserve to be discussed, since they had a strong suspicion of SPN on imaging exams. In the first, CT and MRI confirmed the diagnosis of pseudocyst, the EUS suspected SPN, and the EUS-FNA revealed NF-NET, which was confirmed by uncinate process resection. The second had CT, MRI, and EUS image exams with SPN morphology (solid-cystic), the McH obtained by EUS-FNA confirmed the presence of SPN, but the operative piece confirmed NF-NET. In this case, the diagnosis by McH confirming SPN was performed only by HE, as there was not enough material to perform the IHC. These findings corroborate those found in the literature where the EUS-FNA performed on suspected SPN has excellent positive and negative predictive value; however, the most common classification errors were with NF-NET which presents no clinical impact, just as it was observed in our series [7]. In addition, in 177 patients studied by the EUS-FNA with suspected NF-NET, discrepancies were reported in 14 patients. In 4 of them, the erroneous diagnosis was SPN. Accordingly, it is concluded that when an adequate sample is obtained, the most significant error is the incorrect classification, which is more often associated with SPN, but the damage associated with this diagnostic error is minimal [24].
The authors conclude that SPN are rare, incidentally identified in most cases and affect young women. Isolated CT, MRI/MRCP, and EUS have low rates of correct diagnosis, but the association with EUS-FNA increases pre-operative diagnosis and should be used whenever necessary to rule out other pancreatic diseases such as NF-NET, SCA, or other type of solid/cystic nodular lesion of the pancreas. These results suggest that patients who do not have a clear diagnosis of SPN, as is usually the case, should undergo EUS-FNA.















