Introduction
Oesophageal cancer is the seventh most incident globally and the sixth in terms of mortality, with a mortality/incidence ratio of 0.89 [1]. It belongs to the cancer types with the highest risk for weight loss and malnutrition [2] and nutritional status is expected to further worsen with multimodal treatment [3]. In fact, nutritional intervention may lead to an improved treatment tolerance for patients receiving chemoradiation [3], while those who experience severe preoperative weight loss face a higher 5-year mortality rate after oesophagectomy [4].
Nutritional risk in these patients can potentially be ameliorated by the placement of a percutaneous endoscopic gastrostomy (PEG) tube. Some studies, most of them retrospective, have evaluated the benefits of PEG-tube placement in oesophageal cancer patients before multimodal treatments. Although some demonstrated better nutritional management in these cases, most had equal results in terms of perioperative complications, tolerance to chemoradiotherapy (CRT), and overall survival [3, 5-8]. Additional concerns regarding PEG-tube placement are the risk of tumour cells seeding in the stoma and affecting gastric vascularization, thereby precluding surgeons from using a gastric conduit after oesophagectomy, which is the reason why international guidelines so far advise against percutaneous gastrostomy in surgical patients [9]. Nevertheless, evidence suggests that these are rare events [5, 7, 8, 10].
Comparing PEG with other nutritional methods, it is less invasive and is associated with lower complication rates than jejunostomy [11]. Nasogastric tubes usually have lower functional and aesthetical tolerability in mid to long term [12] and, regarding endoscopic stents, although effective in improving dysphagia, it has not been demonstrated that these are effective in improving nutritional status [13-16] and may even be associated with a high complication rate [17].
The primary goal of this study was to assess the impact of PEG-tube placement on the nutritional status of patients with oesophageal cancer requiring definitive or neoadjuvant CRT. Secondary outcomes were (i) to evaluate procedure safety; (ii) to assess feasibility of oesophagectomy with a gastric conduit in these patients; (iii) to assess the risk of stoma tumour seeding.
Materials and Methods
A comparative study with a prospective arm (2016-2020) and a retrospective historical control cohort (2008-2010) was conducted in a Portuguese oncological centre.
Population
Patients aged 18 or higher with oesophageal cancer (squamous cell carcinoma or adenocarcinoma) or oesophageal-gastric junction cancer Siewert I (adenocarcinoma) with dysphagia grade >2 and/or weight loss >10% of body weight requiring isolated or neo-adjuvant CRT were proposed for PEG-tube placement on a weekly multidisciplinary meeting, prior to the onset of therapeutic protocol (1-3 weeks before CRT, based on the clinical discretion of the treating physicians). Exclusion criteria included contraindication to PEG-tube placement (such as incapacity of handling the PEG tube, decompensated chronic liver disease, or presence of ascites with a clinical significance).
The study protocol was approved by the hospital’s Ethical Committee (Unidade de Investigação Clínica) with the approval number UI/1011 and registered in the Australian New Zealand Clinical Trials Registry platform with the trial number AC-TRN12616000697482. All patients provided written and oral informed consent to participate in the study and for publication of the results at least 48 h before the procedure.
The control group (CG) was a historical cohort of patients with the same characteristics (grade of dysphagia, weight loss, treatment with CRT) from existing records of oesophageal/oesophageal-gastric junction cancer patients, with first evaluation from January 2008 until December 2010. They were selected orderly starting with the most recent, in order to prevent selection bias, regardless of nutritional interventions during follow-up. No records were excluded.
Sample Size Calculation
Since this was a pilot study, there were no data available on the likelihood of finding positive results, so, according to the central limit theory which states that the sample distribution will be nor-mal or almost normal if the sample size is large enough, generally assuming that a sample size of 30 is considered appropriate, it was assumed that it would be necessary to include 30 patients in the study.
PEG-Tube Placement Procedure
The PEG tube was placed by two gastroenterologists using the pull method, with antibiotic prophylaxis with intravenous cefazoline (2 g) administered immediately before the procedure. Standard gastroscopes were used (Olympus series 165 or 190). If the oesophageal tumour caused an unsurpassable stenosis with the en-doscope with the smallest diameter available (ultra-thin endoscopes were available only in the second half of the study period), an oesophageal dilation was also performed by an experienced gastroenterologist, using a Savary-Gilliard® dilator (9 mm) or a hydrostatic balloon (10 mm). After PEG insertion, a peristoma swab was collected, with subsequent cytopathological evaluation. After the procedure, the patient was hospitalized for surveillance for 24 h and started enteral bolus 3 h post-procedure, if no complications occurred. Throughout the course of treatment, the patient could maintain oral diet in addition to enteral nutrition. A nutritional plan was established by a dietitian. In patients undergoing oesophagectomy, the PEG tube was removed during surgery and the peristoma tissue was sent for histopathological evaluation by a pathol-ogist with expertise in digestive pathology. In non-surgical patients, the PEG was removed at the end of the treatment if oral nutrition was possible.
Chemoradiotherapy
According to the institution’s protocol, T2-4 or N+ patients unfit for surgery, T3-T4a or N+ patients with conditions for surgery, and any patient with cervical oesophageal cancer undergo CRT with two cycles of 5-FU combined with cisplatin and radiotherapy (50.4 Gy), which may be personalized by the attending oncologist. These patients are restaged after 4-6 weeks and the final decision to undergo surgery or proceed to definitive CRT is made.
Follow-Up
Nutritional outcomes, surgical complications, urgent hospital admissions, and any complication related to the PEG were registered. Nutritional status was evaluated using body mass index (kg/m2). According to the hospital’s protocol for oesophageal cancer patients, they have a first nutritional evaluation by the dietitian at diagnosis, at least twice during CRT (more often in case of increased nutritional risk), and after treatments. These records were used for the CG and were complement by medical records whenever necessary, since weight and dysphagia were systematically documented in the Oncology appointments, which occur every 2 weeks during CRT. In the intervention group (IG), patients’ weight was also registered by a Gastroenterology Nurse (see later) every 2 weeks before CRT, weekly thereafter, at weeks 2 and 4 after CRT, and 1 month after surgery.
PEG-related complications were assessed according to type of complication, approach and severity (medical - minor, endoscopic or surgical - major adverse events [AEs]), and time (immediate - until 24 h after procedure, early - less than 7 days, late - 7 days or later). All the records were made by a nurse with more than 5 years of experience in the management of patients with gastrostomies. Follow-up was completed 6 months after surgery or definitive CRT.
Statistical Analysis
For statistical analysis, SPSS Statistics 26 (IBM) was used. Demographic and clinical characteristics were presented as frequencies. Continuous variables were expressed as average and standard deviation or as median and interquartile range, according to data distribution, and were compared using t-Student or Wilcoxon tests, respectively. Qualitative variables were compared using χ2 or Fisher exact tests. A p value lower than 0.05 was considered statistically significant.
Results
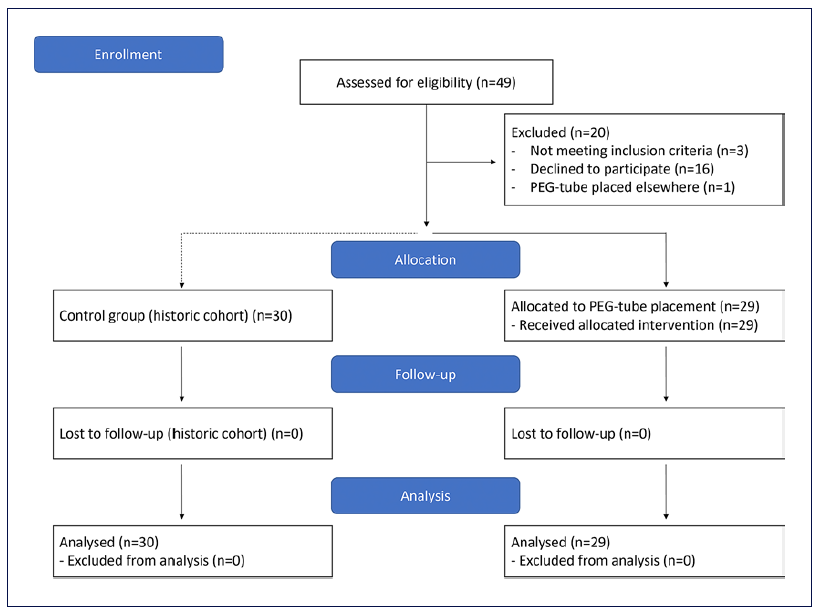
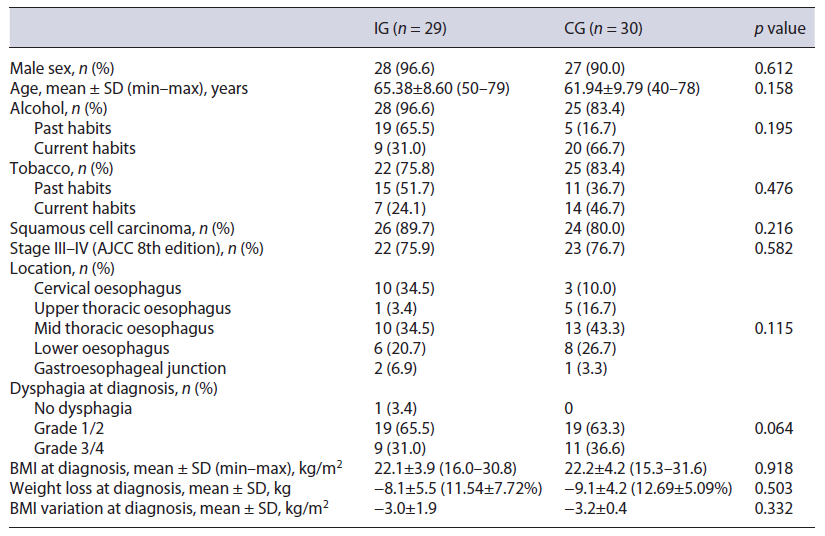
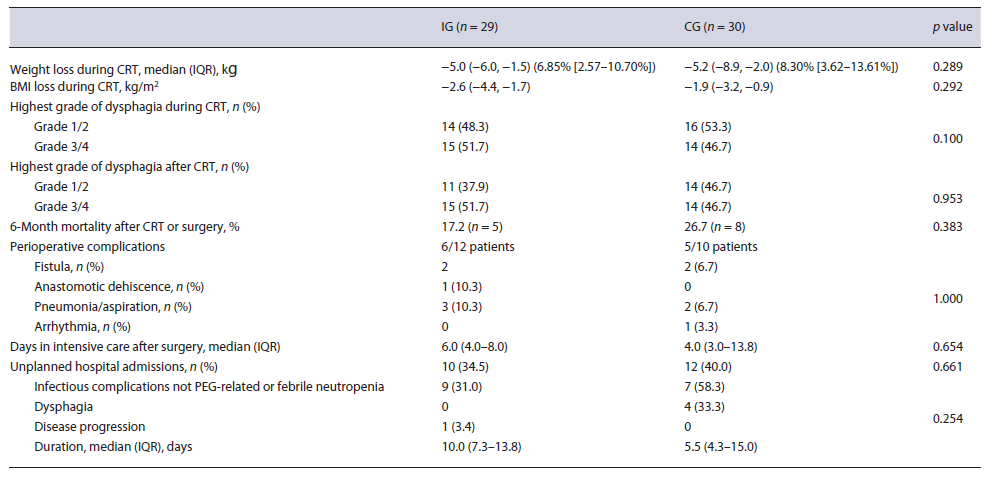
Forty-seven patients were considered for the trial, of which 29 were included (IG) (Fig. 1). The initial goal of 30 patients was not achieved because of the time allocated for the study. These were compared to 30 patients from the historic cohort (CG). Both groups were similar regarding demographic and clinical characteristics at base-line. In the IG and in the CG, there were mainly males, aged above 60 years old, with significant past or active alcoholic habits. The most common diagnosis was squamous cell carcinoma, frequently diagnosed in advanced stages. A weight loss higher than 10% was frequently found and dysphagia grade at diagnosis was higher than 2 in over a third of the cases. Table 1 describes patients’ characteristics in detail.
In the CG, before CRT, 10 (33.3%) patients required oesophageal dilation and 2 (6.7%) enteral feeding by the nasogastric tube. During CRT, 14 (46.7%) patients presented with dysphagia grades 3 or 4, 12 of them with the need of additional nutritional therapy: nasogastric tube feeding (n = 10), surgical gastrostomy (n = 1), and oesophageal dilation (n = 1).
In the IG, PEG-tube placement success rate was 100%. Pre-procedural oesophageal dilation was necessary in 11 patients (37.9%). 89.3% patients used the PEG tube during CRT, exclusively at some point in 50%. The procedure was mainly associated with minor AEs (n = 12, 42.9%) and 1 major (exploratory laparotomy due to suspected colonic interposition, not confirmed). Minor AEs were managed conservatively and included bleeding (n = 2, 6.9%), which occurred in the first 24 h after procedure and peristomal infection (n = 10, 34.5%) which was diagnosed early (24 h to 7 days) after procedure in 3 patients and later in 7 cases. In those who underwent surgery (n = 12), technical success was not affected by PEG and it did not influence the type of surgery nor implied the use of colon as a conduit after oesophagectomy. There was no evidence of cytological or histological evidence of tumour seeding in the stoma.
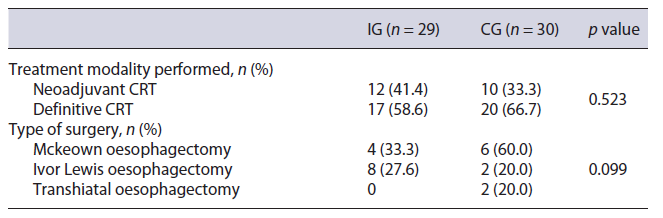
After initial staging, neoadjuvant CRT was proposed to 55.2% (n = 16) of the IG and 60.0% (n = 18) of the CG (p = 0.708), but the strategy was changed to definitive CRT in 4 patients of the IG and 8 of the CG (Table 2). This was due to other comorbidities (IG, n = 2; CG, n = 1), disease progression (IG, n = 2; CG, n = 6), and patient’s choice (CG, n = 1). Body mass index variation during CRT was similar between groups (median and IQR in the IG: −2.6 [−4.4, −1.7] and in the CG: −1.9 [−3.2, −0.9], p =0.292) (Fig. 2), as were 6-month mortality after surgery or CRT (IG 17.2% and CG 26.7%, p = 0.383), perioperative complications (IG 54.5% and CG 55.6%, p = 1.000), and unplanned hospital admissions (IG 34.5% and CG 40.0%, p = 0.661) (Table 3).
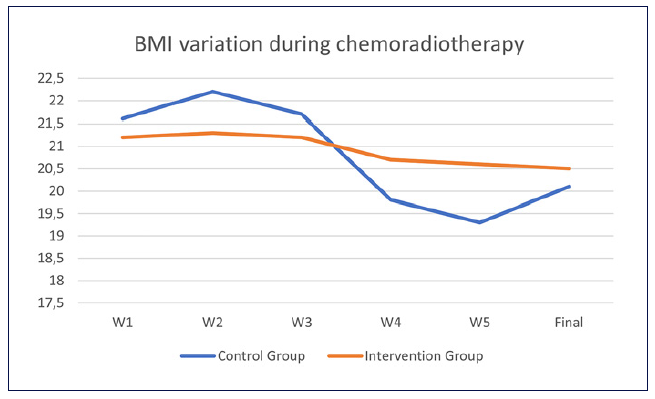
Fig. 2 Weight variation during chemoradiotherapy (CRT). Y axis, BMI (kg/m2); X axis, week since the start of CRT.
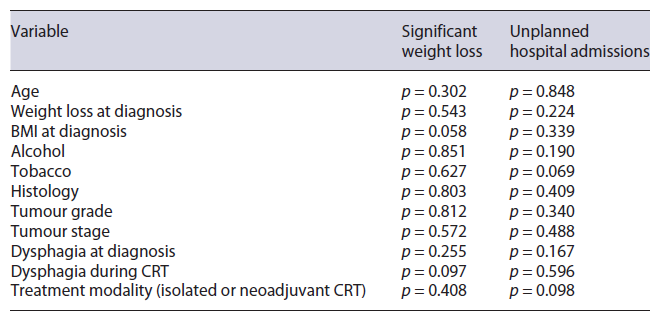
In the univariate analysis, significant weight loss during CRT, defined as a weight loss of at least 10% during this period, was not associated with age (p = 0.302), alcohol (p = 0.851) or tobacco (p = 0.627) consumption, weight loss at diagnosis (p = 0.543), histological type (p = 0.803), tumour grade (p = 0.812), stage (p = 0.572), dysphagia grade at diagnosis (p = 0.255) or during CRT (p =0.097). Similarly, unplanned hospital admission did not show a significant association with these variables (Table 4).
Discussion/Conclusion
In patients with oesophageal cancer treated with definitive or adjuvant CRT, prophylactic PEG-tube placement did not improve the most relevant clinical outcomes, namely, weight loss during CRT, unplanned hospital admissions, surgical complications, and mortality.
This was in line with the findings of retrospective studies such as that of Bhatti et al. [7], as well as others with smaller samples [5, 6, 10]. Nevertheless, as depicted in Figure 2, weight variation was more pronounced in the CG, with possible metabolic implications, since weight fluctuation might be associated with an increased risk of all-cause mortality, as shown in the general population [18].
Comparing both groups, it was striking that almost half of the patients who were not submitted to PEG-tube placement before CRT required enteric nutritional support during treatment, which usually consisted of nasogastric tube feeding. On the other hand, most of the patients with prophylactic PEG used it complementarily and, in half of the cases, as an exclusive way of nutrition at some point during CRT.
PEG-tube placement was a feasible procedure, with minor AEs in around 40% of the cases, mostly late peristomal infections. However, 1 patient needed an exploratory laparotomy because colonic interposition was suspected but not confirmed. Infectious complications are already known to be the most common PEG-associated AEs [19, 20]. Zopf et al. [19] conducted a prospective multicentre study including 390 patients, where 33.6% had peristomal infection and the presence of malignant disease had a significant association with this risk, which may explain our findings. This complication rate is nonetheless higher than that reported in other studies such as that conducted by Margolis et al. [8], in which 103 patients with oesophageal cancer and PEG-tube placement were retrospectively evaluated. It is not clear whether this difference is due to technical differences, bias associated with the study type, or other reasons. Whether the high rate of peristomal infection is also attributable to the technique chosen (pull vs. “push”/gastropexy) is not clear since there are discordant reports comparing these groups [19, 21, 22].
Importantly, the PEG tube did not affect type of surgery or surgical technical success, as reported in other studies [5, 7, 10]. Matsumoto et al. [5] further consolidated these conclusions through intraoperative thermal imaging, which demonstrated that gastric blood flow was not affected by previous PEG.
Recently published guidelines from the European Society of Gastrointestinal Endoscopy recommend the percutaneous introducer (“push”) technique for PEG placement in patients with head and neck cancer or oesophageal cancer [23, 24]. The main reason underlying this recommendation is the risk of metastasis to the PEG site. The meta-analysis from Siu et al. [25], including 121 cases, reported rates of 0.56% (95% CI: 0.40-0.79%) with the “pull” technique and 0.29% (95% CI: 0.15-0.55%) with the “push” technique. This difference is not neglectable and must be considered, despite the null rate of stoma tumour cells found in our study. It is also noteworthy that even in the “push” group, PEG site metastasis was found, supporting haematogenous or lymphatic spread of the tumour cells, an important mechanism, as already suggested in the literature [26, 27].
A notable advantage of this study was its prospective nature, which allowed a precise and unbiased data collection in the intervention arm. This was performed in a hospital with expertise in Oncology, where the best standard-of-care was applied, allowing an extrapolation of the results.
There are important limitations to mention, namely, the retrospective evaluation of the CG. However, since the patients in the historic cohort had both medical and nutritional follow-up, the records were very complete and possible errors in retrospective data collection were mitigated. It is also worth noticing that a small sample was used and it is possible that the study may have not been adequately powered. Other nutritional endpoints such as hand grip strength and albumin were not systematically assessed and were not included in our results, further limiting conclusion withdrawal. It would have been interesting to know the nutritional status of these patients 6 months post-CRT, but this variable was not foreseen, thereby not prospectively collected and not included due to the risk of collecting biased information.
In conclusion, this study strengthens current knowledge about prophylactic PEG-tube placement in oesophageal cancer patients undergoing multimodal treatment. This is a safe procedure, with predominantly minor AEs and no evidence of tumour cells seeding in the stoma in the population studied. One of the main advantages of this intervention is avoiding invasive procedures aiming for enteral feeding during CRT.