Introduction
In the diagnostic workup of obscure gastrointestinal bleeding (OGIB), defined as bleeding from the digestive tract with negative findings on upper endoscopy and colonoscopy, small-bowel capsule endoscopy (SBCE) remains the first-line diagnostic tool in haemodynamically stable patients [1, 2]. SBCE has a well-documented diagnostic yield and positive impact in the management of these patients [3, 4].
Small-bowel vascular lesions, including angioectasia (AE), Dieulafoy’s lesion and arteriovenous malformation are the most common causes of OGIB [5]. AE are found in 30-40% of patients presenting with OGIB [6]. Since these vascular lesions are considered to be venous [7], they usually manifest as iron deficiency anaemia (IDA) due to chronic bleeding. However, these lesions may also result in overt bleeding episodes (OBE) [8].
Although being incompletely understood, the pathophysiology of AE includes high intestinal wall tension causing chronic obstruction of the submucosal veins with consecutive precapillary dilation, mucosal ischaemia from chronic hypoxia and vascular endothelial growth factor (VEGF)-related disorders of angiogenesis [9].
As such, numerous conditions have been associated with the presence of these lesions, including vascular comorbidities and ageing [10-12]. In a systematic review conducted by Grooteman et al. [13], which included 92,634 participants from 23 studies, age, chronic kidney disease (CKD) and cardiovascular disease were the most important risk factors for the presence of AE on endoscopy.
The clinical significance of these gastrointestinal lesions is incompletely understood [6], with some studies questioning their impact on survival [14]. In fact, most of these lesions do not require treatment, unless they become symptomatic [15]. In this study, the authors aimed to evaluate the independent effect of small-bowel AE on survival of patients with OGIB.
Methods
Study Design
This was a single-centre, retrospective cohort study.
Patient Population
All adult patients undergoing SBCE between 2010 and 2013 for OGIB, presenting as OBE or IDA, from Centro Hospitalar de Vila Nova de Gaia/Espinho, EPE, were included in this study. The CE system used was Mirocam (IntroMedic, Seoul, Korea). All CE studies were analysed by an expert gastroenterologist with extensive experience in CE (more than 300 CE examinations).
The authors recorded the presence or absence of small-bowel AE (P2 lesions according to the Saurin Classification [16]), num-ber of AE, maximum size of AE and referral for argon plasma coagulation (APC).
Exclusion Criteria
Inadequate small-bowel cleansing and incomplete exams were exclusion criteria from the study. Using the Brotz preparation scale, an exam was considered inadequately prepared if a quantitative index lower than 7 was described [17].
An exam was considered incomplete if the capsule did not reach the cecum/colon or stoma bag (in patients who had had ileocolonic resection or other relevant surgery) during recording time.
Bowel Cleansing Protocol
All patients took the same bowel cleansing procedure: clear liq-uids diet on the day prior to the procedure and 2L polyethylene glycol solution the night before the exam. Four hours after the capsule was swallowed, they were instructed to take a small meal.
Population Characteristics
On the date of SBCE performance, gender and age were recorded, as well as the presence or absence of the following vascular comorbidities (diagnosed before SBCE performance or during follow-up): congestive heart failure (CHF), heart valve disease, ischaemic heart disease, atrial fibrillation, history of previous stroke, abdominal aorta aneurism, peripheral artery disease, history of previous mesenteric ischaemia and CKD.
Referral for APC
In the study centre, a conservative approach for APC treatment of small-bowel AE was used; it is generally reserved for patients who present with a low haemoglobin level and with relapsing anaemia despite iron supplementation.
Follow-Up
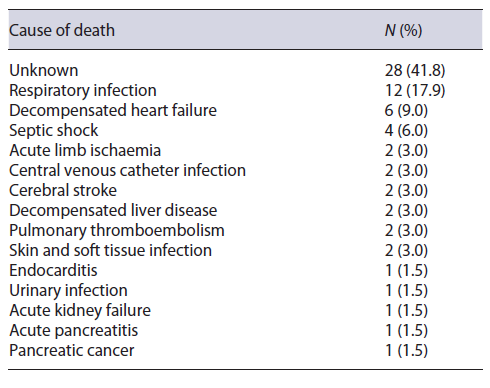
Follow-up started after SBCE and ended upon death or end of the study (November 2020). The primary endpoint of the study was mortality. The date of death was electronically available in all patients who died during follow-up. The cause of death when know (only in patients who died in the study centre facilities) was also reported.
Statistical Analysis
Data was reported as median (interquartile range [IQR]) or mean (standard deviation [SD]), when appropriate, for numerical variables and as absolute and relative frequencies for categorical variables.
The Student t test and the Mann-Whitney test, when suitable, were used to compare numerical variables, and the χ2 test and Fisher’s exact test, when suitable, were used to compare categorical variables.
The Kaplan-Meier method was used to estimate 1-year and 5-year cumulative survival in both groups, and the log-rank test was used to assess differences in overall survival.
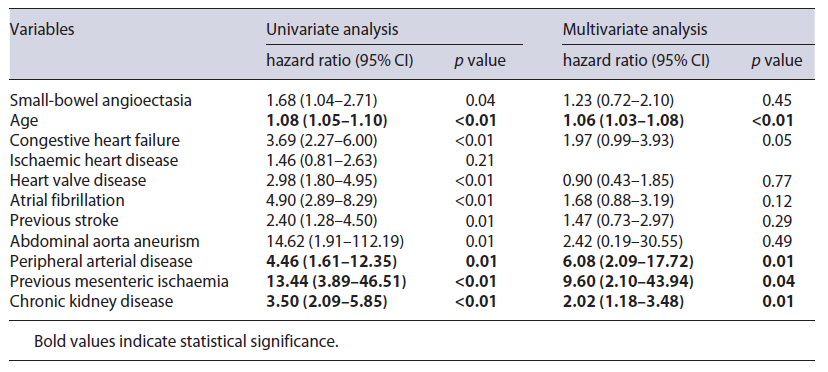
Survival analysis was performed using a Cox proportional-hazards model to analyse the effect of small-bowel AE on survival as well as potentially confounding factors, including age and the comorbidities under evaluation. We initially performed a univariate analysis. Variables with a p value less than 0.1 were included in the multivariate analysis.
We also performed a survival analysis, again using a Cox proportional-hazards model, in patients with AE on SBCE, to assess the effect of age, clinical presentation (OBE vs. IDA), number of AE, AE maximum size and treatment with APC by balloon-assisted enteroscopy on survival.
In order to assess the effectiveness of AE endoscopic treatment, a 2-year follow-up of the patients submitted to APC was carried out. The authors assessed the lowest haemoglobin level, the transfusion support rate and the rebleeding rate (defined as the need for a blood transfusion, the presence of OBE or a decrease in haemoglobin ≥2 g/dL).
The IBM Statistical Package for the Social Sciences (SPSS) version 26 was used for statistical analysis. A p value less than 0.05 was considered as statistically significant.
Results
Between 2010 and 2013, 196 SBCE due to OGIB were performed. The authors excluded 20 of the exams due to inadequate bowel cleansing (n = 13) and incomplete examination (n = 7).
The authors included 176 patients in this study (50.6% male), with a median age of 68.5 years (IQR 24). Regarding exam indication, in 149 (84.7%) of these patients SBCE was performed due to IDA and in the remaining 27 (15.3%) patients due to OBE.
Concerning findings on SBCE, in 122 (69.3%) of SBCE at least one relevant finding was identified: small-bowel AE, 73 (41.5%) exams; erosions/ulcers, 29 (16.5%); mucosal erythema, 10 (5.7%); subepithelial lesions, 5 (2.8%); polyps, 2 (1.1%); haemangioma, 1 (0.6%); Meckel’s diverticulum, 1 (0.6%) and small-bowel tumour, 1 (0.6%).
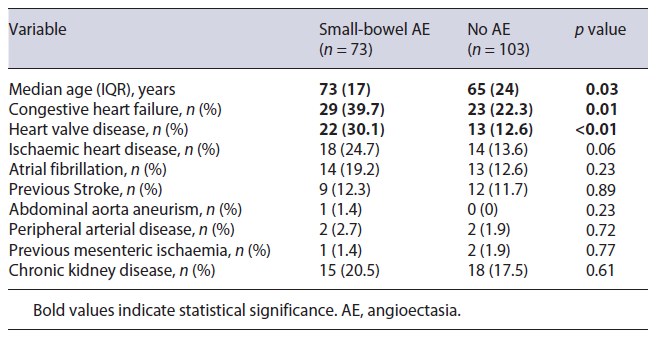
Age and comorbidities were compared between patients with AE and patients without these findings (Table 1). Patients with small-bowel AE were older (73 [IQR 17] vs. 65 [IQR 24] years, p = 0.03) and were more likely to have CHF (29 [39.7%] vs. 23 [22.3%], p = 0.01) and HVD (22 [30.1%] vs. 13 [12.6%], p < 0.01). There were no statistically significant associations concerning the remaining factors analysed.
Table 1 Comparison of demographical data and comorbidities between patients with small-bowel AE and patients without this finding on small-bowel capsule endoscopy
Survival Analysis
The median follow-up was 7 years (IQR 4), during which 67 (38.1%) patients died. Causes of death are reported in Table 2. In 41.8% of the patients, the cause of death was unknown or not reported on the available medical records. In all of the cases of death with known cause, overt small-bowel gastrointestinal bleeding was not reported.
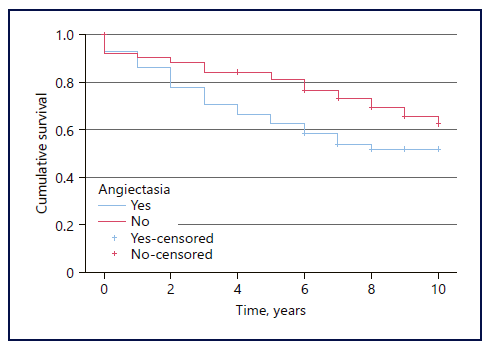
The 1-year and 5-year cumulative survival in patients with AE was 93.2% and 66.7%, respectively, and 92.2% and 84.4% in patients without AE. Overall survival was inferior in the group of patients with AE (p = 0.03) (Fig. 1).
On univariate Cox proportional-hazards analysis, age, the presence of AE and all the analysed comorbidities (except for ischaemic heart disease) were significant risk factors for death. However, on multivariate analysis, only age, peripheral artery disease, history of previous mesenteric ischaemia and CKD were independent risk factors for death. The presence of small-bowel AE per se did not affect survival in this analysis (Table 3).
Subanalysis of Patients with AE on CE
As mentioned before, 73 patients had small-bowel AE. 50.7% of these patients were male and the median age was 73 years (IQR 17). The median follow-up was 7 years (IQR 6), during which 33 patients (45.2%) died.
Regarding indication, 62 (85.3%) of the SBCE were performed due to IDA, whereas the remainder were due to OBE. Concerning AE features, the median number of AE was 3 (IQR 3), the median maximum size was 4 mm (IQR 4), and 28 (38.4%) were submitted to APC.
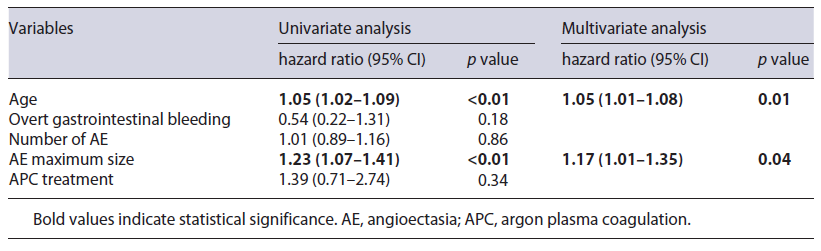
Multivariate Cox regression analysis revealed that age and AE maximum size were independent predictors of poor survival, whereas indication for SBCE, number of AE and APC treatment were not (Table 4).
Table 4 Comparison of clinical features of patients with AE submitted to argon plasma treatment and patients managed conservatively
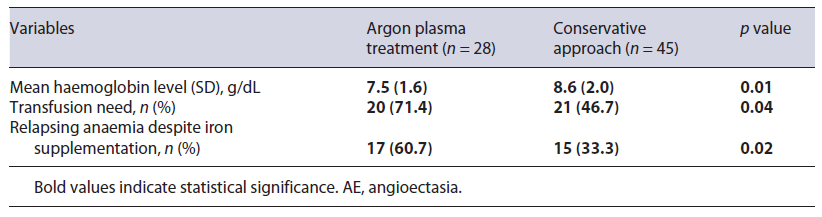
However, when comparing patients who were submitted to APC with those who were not, the first group had a lower haemoglobin level upon OGIB diagnosis. Furthermore, these patients had a higher need for transfusion support and a higher rate of relapsing anaemia despite iron supplementation (Table 5).
Table 5 Comparison of clinical features of patients with AE submitted to argon plasma treatment and patients managed conservatively
Subanalysis of Patients with AE Who Underwent Endoscopic Treatment
On a 2-year follow-up, 4 from the 28 patients submitted to APC died; none of these reached the endpoint of rebleeding. Concerning the remaining 24 patients, the rebleeding rate was 37.5%. However, the mean lowest haemoglobin level was significantly higher than the value upon OGIB diagnosis (10.2 [0.4] g/dL vs. 7.5 [1.6] g/dL, p < 0.01), and the transfusion need was significantly lower (5 [20.8%] vs. 20 [71.4%], p < 0.01). Furthermore, there were no OBE reported during this period.
Discussion
IDA and OBE, two possible clinical presentations of small-bowel AE, are factors which may decompensate underlying comorbidities. In fact, the presence of anaemia in patients with CHF increases overall mortality and hospitalization rate according to a recent meta-analysis [18].
However, when analysing the independent effect of AE on survival, the authors did not find a significant association. The authors also did not find any recorded death directly associated with AE. These findings are similar to a recent study published by Robertson et al. [14], in which small-bowel AE did not cause any death in a 5-year cohort, but survival was poor in the AE group due to the higher frequency of vascular comorbidities.
Concerning the subanalysis of factors impacting survival in patients with AE, besides age, only AE size had an independent effect on survival. It is plausible to affirm that a larger number of participants would be necessary to strengthen the conclusion of this subanalysis, given that multiple lesions, for instance, increase the risk of rebleeding and, therefore, there is an expected benefit of treating these patients [13, 19, 20].
Although APC treatment was not identified as an independent predictor of survival, it is important to consider that in the study centre, there is a conservative approach for endoscopic referral. In fact, the group of patients submitted to APC had a lower haemoglobin level upon OBE diagnosis, a higher need for transfusion support and a higher rate of relapsing anaemia. These considerations may explain the absence of survival impact of APC treatment in the study analysis, given that a conservative approach was taken in patients with a better prognosis and the invasive approach was reserved for patients with more adverse clinical features upon OBE presentation.
Concerning the effectiveness of the procedure, although more than one-third of the patients fulfilled the criteria of rebleeding, the haemoglobin level significantly improved, as well as the transfusion need, and no OBE were reported. Moreover, the increase in the mean haemoglobin level and the decrease in transfusion support achieved with endoscopic treatment are indirect markers of quality of life improvement and reduced utilisation of healthcare services [21].
The authors present a real-world analysis of a population with multiple comorbidities, which have a stronger impact on survival than the presence of AE. Despite the neutral effect on survival, it is important to keep the high rebleeding rate of these lesions after endoscopic treatment in mind (especially in patients with high-risk comorbidities), as well as the eventual need for a second or even third enteroscopy [22, 23].
Nevertheless, with these considerations, the authors do not aim to discourage the treatment of these lesions, but rather to alert for the need to outweigh its risks and benefits, especially in older and frail patients with IDA compensated with iron supplementation. This study has several strong points. Firstly, the median follow-up of this study is quite large: 7 years (IQR 4). Secondly, to our knowledge, this is the first study which analyses the independent effect of small-bowel AE, adjusted for age and vascular comorbidities, using a multivariate Cox regression model. Thirdly, the authors only included P2 AE according to the Saurin classification, excluding red spots (P1 lesions), which do not have a significant impact in this setting [24].
This study has, however, two important limitations. Firstly, data was collected retrospectively through the medical records of the study centre; therefore, the authors were only able to know the cause of death of patients who died in the hospital facilities. Secondly, other potential risk factors for the presence of AE, which might affect survival, were not analysed, including obesity and chronic liver disease [9, 25].