Introduction
Short-bowel syndrome (SBS) is a rare disease, with an estimated European prevalence of 1-9 cases per 100,000 inhabitants, which arises from a context of extensive bowel resection, congenital defects, or underlying disease, leading to the loss of absorptive intestinal surface [1, 2]. Generally, SBS is defined as small bowel length of less than 200 cm, with residual small bowel of less than 10 cm in children or 20 cm in adults known as ultra-SBS. However, the absorptive limitations reflect not only the extent of the residual small bowel length but also the anatomy, functionality, and adaptative potential of the remaining intestine and patient clinical condition [2-6]. Home parenteral nutrition (HPN) represents the standard-of-care and life-sustaining therapy in patients with SBS and chronic intestinal failure (SBS/CIF) [7, 8].
Treatment-related complications are reported to account for around 14% of the total of deaths in patients with CIF [9]. Long-term HPN complications include central venous catheter (CVC) problems, particular catheter-related blood stream infections (CRBSIs) responsible for over 70.0% of hospitalizations in HPN patients, and met-abolic complications, of which intestinal failure-associated liver disease remains a major cause of patient morbidity and mortality [8, 10, 11]. Albeit the benefits in patient longevity and nutritional status, HPN is associated with quality-of-life (QoL) deterioration [12, 13], with SBS/CIF patients showing lower QoL scores when compared to the general population and other chronic diseases [14].
Costs associated with long-term HPN are not negligible and tend to increase with extension of patient longevity. Although HPN costs are counterbalanced by its life-sustaining nature, economic impact in the healthcare system reflects direct costs associated with PN, medical consultations, laboratory monitoring, home support, and hospitalizations due to treatment-related complications. Additionally, non-healthcare costs and indirect costs, due to productivity loss, also contribute to the overall economic burden [13].
In Portugal, a country with 10 million inhabitants, there is a clear absence of national evidence regarding the real-world context of SBS/CIF patients, and to date, no national study unveiled the multidimensional impact of SBS/CIF. As such, we conducted the PARENTERAL study (ImPActo da SíndRomE do INTEstino Curto em PoRtugAL) with the aim to characterize the clinical, economic, and humanistic impact of SBS/CIF in Portugal.
Materials and Methods
Study Design and Setting
The PARENTERAL study was a nationwide chart review study, with an observational retrospective cohort design and a cross-sectional component to evaluate SBS/CIF patients’ QoL. Investigation center enrollment followed the initial contact with the Portuguese Association of Enteral and Parenteral Nutrition (APNEP) to iden-tify the hospital centers treating SBS/CIF patients.
Study Population
Patient eligibility was assessed according to the following inclu-sion criteria: patients diagnosed with SBS/CIF; age ≥1 year-old; home- or hospital-based PN considered stable, defined as under PN for a period ≥6 months; no previous treatment with teduglutide or any other growth hormone. Pregnant or breastfeeding women, patients diagnosed with active malignant disease, or history of gastrointestinal cancer on the past 5 years were excluded. Additionally, patients or caregivers unwilling or unable to provide informed consent were not eligible for study participation.
Eligible patients or respective caregivers were invited to participate by their attending physician during follow-up visit. The overall recruitment period ranged from March 2018 to September 2019.
Data Collection and Variables
Data were collected through three main instruments: clinical case report form; self-report patient questionnaire; and QoL measurement instruments. After obtaining written informed consent, clinical case report form was filled by the investigator and captured electronic health record data regarding patient demographics and SBS/CIF clinical characterization, therapeutic management (PN support and pharmacological treatment) and healthcare resource use (HRU) (including medical consultations and consultations with other healthcare professionals, home visits, emergency department [ED] episodes, hospitalizations, laboratory and imaging exams, surgeries) collected from chart review over a 12-month observation period.
The patient questionnaire was handed by the investigator during patient follow-up visit and, whenever possible, self-administered or in the applicable cases, by the parent/caregiver. Self-reported data included SBS/CIF-related symptomatology and healthcare- and non-healthcare-related costs, including healthcare products, private healthcare and insurance, transportation, home adaptations, medical devices, and home care. A 6-month recall period was selected to address and minimize the potential of recall bias, chosen according to the available literature evidence regarding recall period length in healthcare surveys and investigators advice [15, 16].
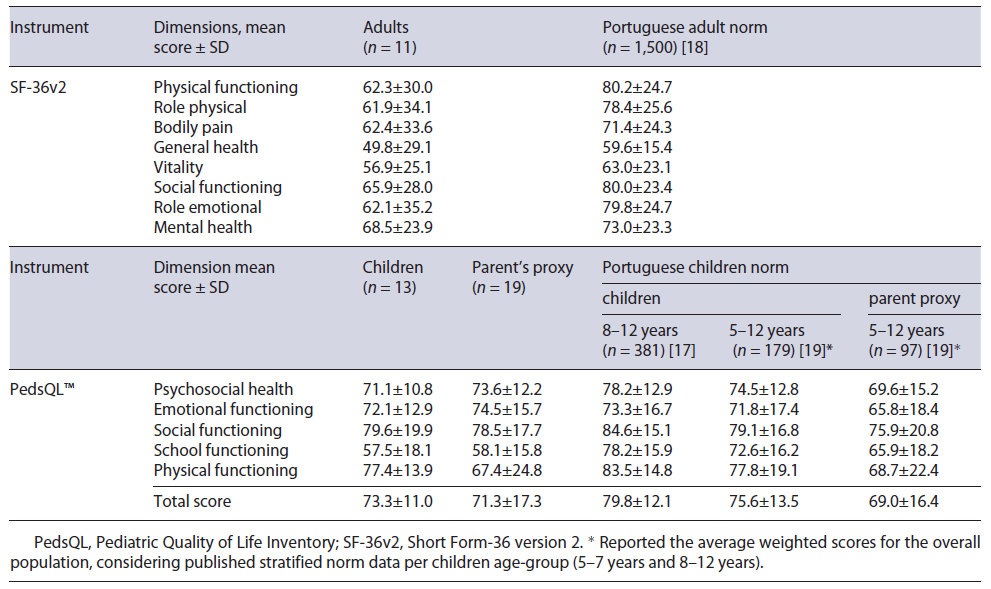
QoL assessment included the Portuguese versions of the Short Form-36 version 2 (SF-36v2) and Pediatric Quality of Life Inventory version 4.0 (PedsQLTM) for adult and pediatric SBS/CIF patients, respectively, both with a 1-month recall period. Both generic instruments were chosen due to validation in Portuguese population and availability of normative data that would allow comparison of QoL scores with the general population or other chronic illnesses [17-19]. As in the self-report questionnaire, instruments were handed to patients by the investigator during follow-up visit and, whenever possible, self-administered.
Healthcare and Non-Healthcare Cost Estimation
Cost analysis focused on direct costs (healthcare and non-healthcare costs), namely, on expenditures supported by the Portuguese National Health System (NHS), patients, and caregivers. Cost estimation included two major steps: identification and classification of cost items; measurement and valuation, i.e., attributing a monetary value to each item. Cost item and cost source are detailed in online supplementary Table S1 (for all online suppl. material, see https://www.karger.com/doi/10.1159/000526059). PN support costs included acquisition cost for commercially premixed ready-to-use PN admixture and tailored PN admixtures formulation (PN bags, formulation nutrients, and supplements).
Data from electronic medical prescriptions regarding inpatient and outpatient pharmacological treatment were retrieved, and costs estimated based on unit prices reported by public NHS hospital tenders and, for retail medication, selling prices in the national medicines database [20, 21]. Healthcare products costs, namely, consumables, were estimated according to available tender documents and retail prices.
Unit costs of healthcare resources, including consultations with other healthcare professionals, home visits, ED episodes, surgeries, and laboratory and imaging exams, were retrieved from national diagnostic-related group tariffs [22, 23]. Hospitalization costs were estimated based on patient’s length of stay (LoS) per specific hospital clinical unit. Average daily costs of hospital stay and medical visit costs, per medical specialty, were retrieved from the analytical elements national database [24].
Out-of-pocket expenses, i.e., nonrefundable healthcare costs directly paid by patients and/or caregivers, were reported by patients and included private insurance, private healthcare, formal home care, medical devices and required home adaptations. Patient costs with transportation were estimated by proxy, considering the unit cost per km in the national tariff for nonurgent patient transportation and travel burden data reported by patients [25].
Statistical Analysis
Study population was described according to demographic and clinical characteristics, through measures of central tendency (mean) and dispersion (standard deviation [SD]) of continuous variables and by absolute and relative frequencies of categorical variables. Cost-related outcomes were estimated through the product of cost item number, frequency, duration, collected from the instruments, and the unitary cost, obtained from official national sources.
Total costs were estimated by a Poisson regression model as a mean annual cost per patient. The mean number of patient monthly travels to the hospital and the respective monthly traveled distance (kilometer) were also estimated by a Poisson regression model. The assumption of cost and utilization homogeneities over the year was used to express annualized costs. Costs were annualized assuming constant use of resources, except for one-off acquisition costs (medical devices and home adaptations), and standardized using the 2019 consumer price index.
QoL assessed by the SF-36v2 score as well as the PedsQLTM was summarized by the calculation of the mean and SD of their main domains and summary or total scores. No data imputation was performed. All statistical analysis adopted a 5% significance level, and whenever applicable, 95% confidence intervals (CIs) were reported. Statistical analysis was performed with the statistical software R 4® [26].
Results
Population Characterization
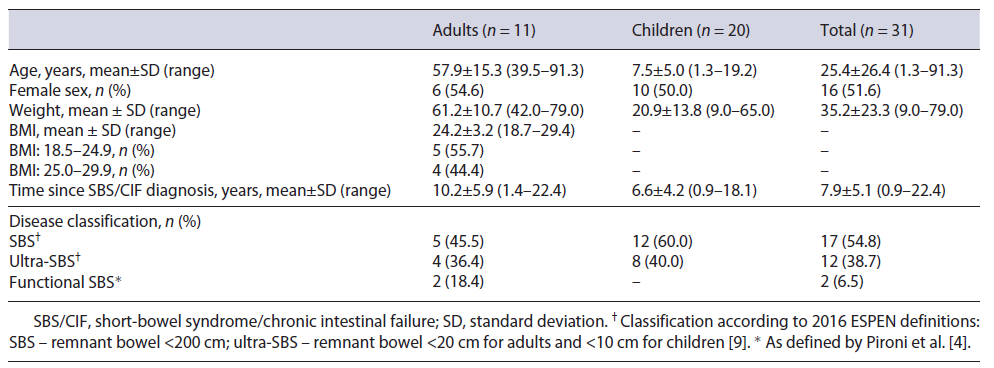
A total of eight hospital centers nationwide were approached, of which six accepted to participate in the study. In the participating centers, 41 patients were assessed by investigators for study eligibility, of which 10 were not eligible for study participation. As such, the study population comprised 31 patients, namely, 11 adult and 20 pediatric SBS/CIF patients (online suppl. Fig. S1). The patient characteristics are presented in Table 1. Patient mean (SD) age was 57.9 (15.3) and 7.5 (5.0) years in the adult and children cohort, respectively, with an even sex distribution.
In the adult cohort, the mean time since diagnosis was 10.2 (5.9) years. All the patients presented CIF, most with bowel length reduction classified as SBS (45.5%) and ultra-SBS (36.4%). The remaining patients (18.4%), despite not having major intestinal resections, had a significant reduction on the gut function. Comparatively, a mean of 6.6 (4.2) years since SBS/CIF diagnosis was reported in the pediatric cohort, with 60.0% of patients classified as SBS and 40.0% with ultra-SBS.
SBS/CIF Clinical Impact
Regarding nutritional support, except for 1 pediatric patient, all patients had oral caloric intake. Enteral nutrition was given in four children, with feeding via surgical gastrostomy (n = 2), percutaneous endoscopic gastrostomy (n = 1), or nasogastric tube (n = 1).
Four adults and three children had at least one type of ostomy, of which jejunostomy was the most common (n = 4). No enterocutaneous fistula was reported among included patients. Overall, 66.6% of patients reported at least one SBS/CIF-related symptom over the previous 6-month period, including diarrhea (46.7%), fatigue/weakness (36.7%), flatulence (33.3%), nausea/vomiting (30.0%), and abdominal pain (26.7%).
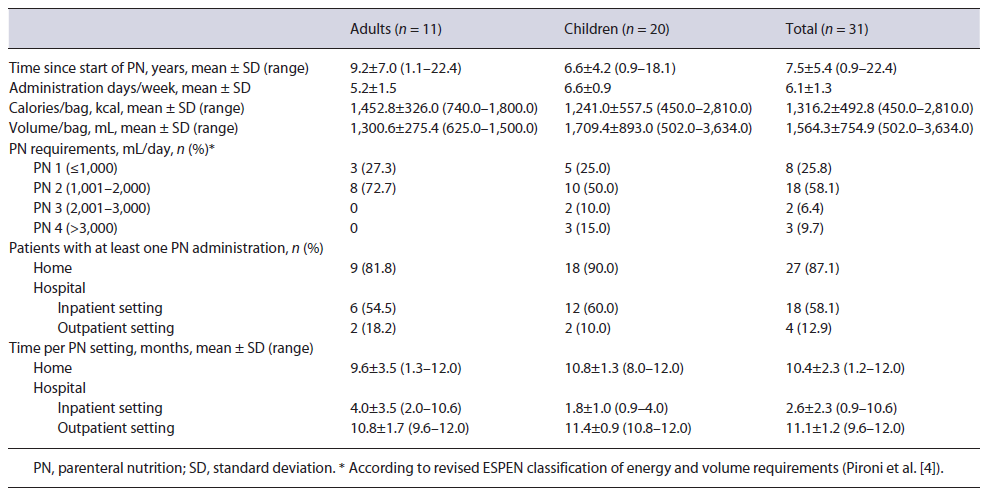
Table 2 summarizes PN support characteristics. PN support spanned for years, with a mean of 9.2 (7.0) years in adults and 6.6 (4.2) years in children since treatment initiation. The average weekly PN frequency was 5.2 (1.5) and 6.6 (0.9) administration days in adults and children, respectively. Regarding the European Society for Clinical Nutrition and Metabolism (ESPEN) clinical classification of PN volume requirements [4], more than half of patients (58.1%) were categorized as PN2 with daily volume ranging from 1,001 to 2,000 mL.
Considering the PN administration setting, 81.8% of adults and 90.0% of children were on HPN during a mean of 9.6 (3.5) and 10.8 (1.3) months over the 12-month observation period, respectively. Nevertheless, 54.5% of adults and 60.0% of children had inpatient PN administration at least once during the observation period, lasting an average of 4.0 (3.5) and 1.8 (1.0) months. Most of the patients (83.9%) were being treated for prevention of catheter-related complications, including the use of taurolidine (adults - 9.1%; children - 68.8%), heparin (adults - 90.9%; children - 25.0%), or both (children - 6.3%).
SBS/CIF Economic Impact
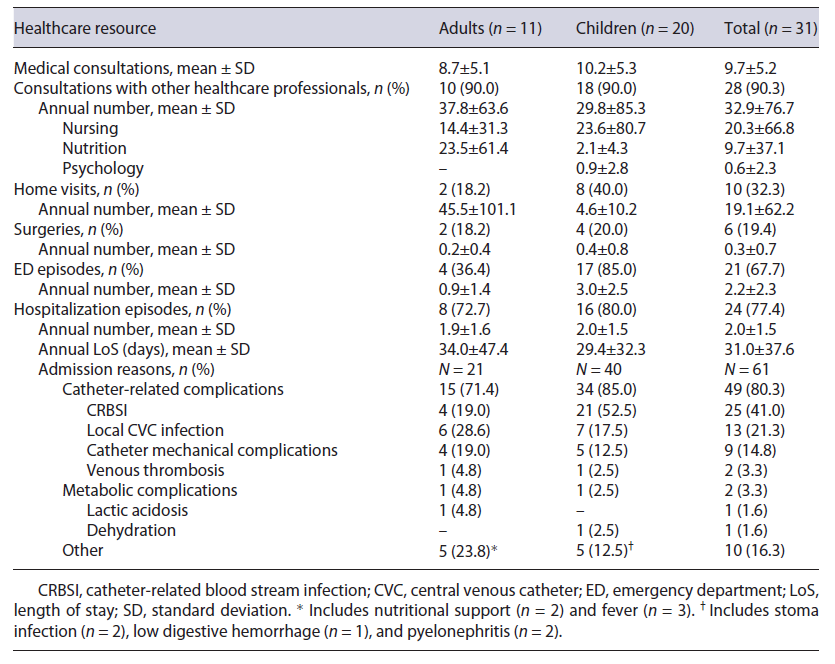
Table 3 presents HRU for SBS/CIF management. During the observation period, an annual average of 8.7 (5.1) and 10.2 (5.3) medical consultations were reported in adults and children, respectively. Patient follow-up with other healthcare professionals accounted for an average of 37.8 (63.6) consultations in adults and 29.8 (85.3) in children during the 1-year period, mainly nursing and nutrition.
Home visit frequency differed widely between patient cohorts. In the adult cohort, only 18.2% of patients required domiciliary visits, although with a significant resource use considering an annual average of 45.5 (101.1) visits. Comparatively, almost half of pediatric patients (40.0%) had at least one home visit during study period, with an average of 4.6 (10.2) visits.
ED episodes were more frequent in pediatric patients (85.0%), with an average of 3.0 (2.5) episodes. Considering hospitalizations, 77.4% of patients had at least one episode related to SBS/CIF. Globally, there were no major differences in the number of episodes and LoS between patient cohorts, with an average of 2.0 (1.5) episodes, and an overall LoS of 31.0 (37.6) days observed during the 12-month period.
Of a total of 61 hospitalization episodes, 21 in adults and 40 in children, 71.4% and 85.0%, were motivated by catheter-related complications, respectively, of which CRBSI constituted the major driver for hospital admission (adults - 19.0%; children - 52.5%). Other catheter-related complications included local CVC infection, mechanical complications, and venous thrombosis, with no major difference between patient cohorts. Admissions due to metabolic complications were residual in both patient cohorts. LoS per hospitalization episode reason is detailed in online supplementary Table S2.
Although the negligible number of surgeries performed during the observation period (n = 7), the majority were associated with catheter-related complications (n = 5), namely, CVC replacements. Only 23.3% (n = 7) of patients reported having resorted to private healthcare, of which 6 were pediatric patients with an average annual number of 2.1 (4.8) private healthcare consultations.
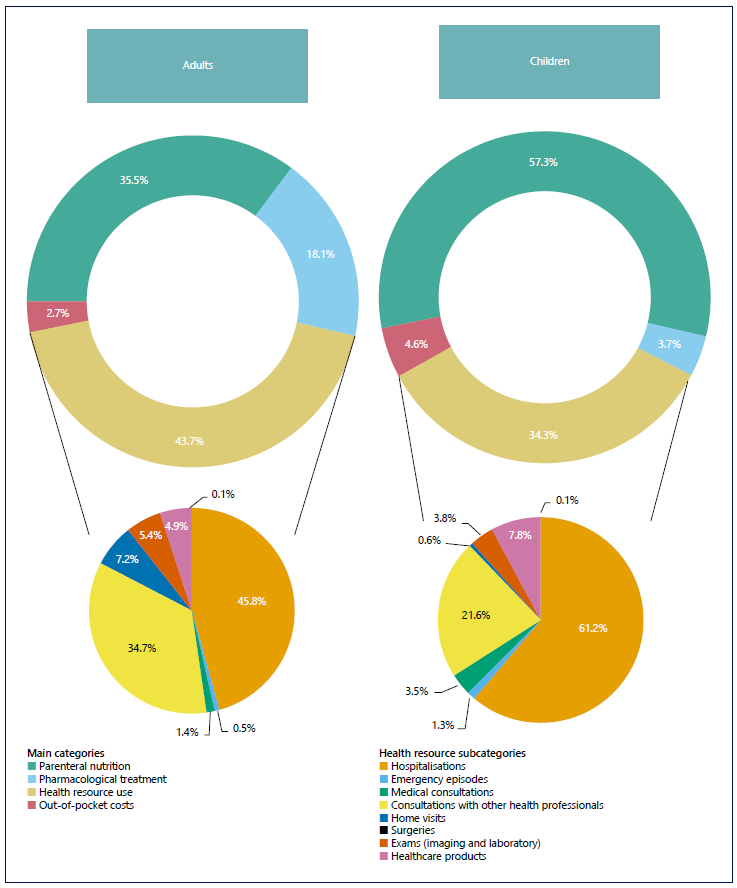
An annual average cost per patient (95% CI) of EUR 65,197.50 (95% CI: 65,017.68-65,287.45) was estimated, namely, EUR 47,857.53 (47,728.43; 47,986.99) in adults and EUR 74,734.49 (74,614.77-74,854.40) in children. Figure 1 presents the distribution of costs in each patient cohort per main cost item categories. The annual average cost per item category is detailed in online supplementary Table S3.
In both cohorts, the main cost-drivers were PN support and HRU, of which hospitalization episodes constituted the major resource. Direct costs associated with PN support amounted to over half (57.3%) of the overall annual cost in the children cohort. HRU costs were higher in adult patients (43.7%), with hospitalization episodes and consultations with other healthcare professionals ascending to more than 80.0% of HRU total cost. For children, HRU constituted 34.3% of the overall annual costs, notably with over half of resource costs associated with hospitalizations (61.2%). Costs related to pharmacological treatment were more substantial in the adult patient cohort, amounting to 18.1% of the annual costs in comparison to just 3.7% in children.
Out-of-pocket costs were residual counting for 2.7% and 4.6% of the overall annual costs in adult and children cohort, respectively. Transportation costs represented 90.0% of the out-of-pocket annual expenses, which is not surprising when considering self-reported travel burden. Patients went to the hospital on average (95% CI) 9.5 times/month (8.4; 10.8), with a higher frequency in children (4.5 times [8.4; 10.8]) when compared to adult patients (3.2 times [2.3; 4.5]).
SBS/CIF Humanistic Impact
Adult Cohort (SF-36v2)
All adult patients filled the SF-36v2 questionnaire. Table 4 summarizes the average raw scores per QoL domain and compares with the Portuguese population norm reported by Ferreira et al. [18]. Globally, SBS/CIF adult patients scored below in every dimension in comparison to the Portuguese general population, with the largest differences found in the physical functioning, role physical, role emotional, and social functioning domains.
PCS and MCS standardized scores, based on Portuguese norm data, are presented in the boxplot (Fig. 2).
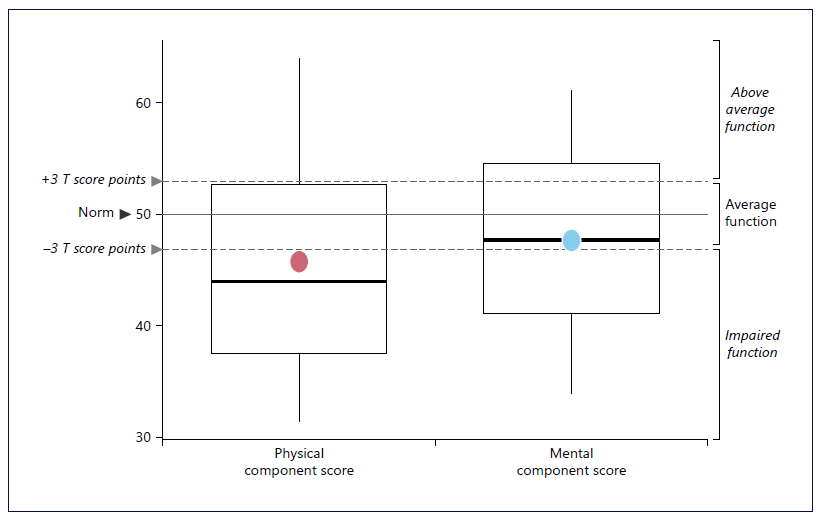
Fig. 2 Standardized physical and mental component scores of the SF-36v2 in SBS/CIF adult patients. Dots represent the mean estimate.
PCS average (SD) score was 45.8 (11.1), i.e., was three SD or t-scores points below norm, which translates into a clinically significant impaired physical function compared to the Portuguese general population. On the other hand, the MCS mean score was 47.7 (8.9), which although being below the Portuguese norm is still considered within the average function interval.
Children Cohort (PedsQLTM 4.0)
Self-report version (n = 14) and proxy version (n = 20) response rates was 92.9% and 95.0%, respectively. The average score per PedsQLTM domain and the Portuguese norm scores from two national studies [17, 19] are reported in Table 4.
The average total score in SBS/CIF children was slightly lower in comparison to the Portuguese norm, with a substantial deterioration in the school functioning domain. In fact, considering stratified data per healthy and chronically ill children (diabetes and spina bifida) reported by Ferreira et al. [19], presented in online supplementary Table S4, average scores in every dimension were lower in SBS/CIF children when compared to the healthy population norm. In line with overall population, school functioning scores were consistently lower even when compared to children with other chronic diseases. In comparison to the Portuguese parent proxy norm, SBS/CIF average scores were higher, except for the school functioning domain.
Discussion/Conclusion
SBS/CIF constitutes a rare, chronic, and debilitating disease, characterized by a complex management and requiring a tailored, multidisciplinary, and comprehensive care. The absence of national evidence regarding the realworld context of SBS/CIF undermines disease awareness within the clinical community, regulators, and overall society, which reflects directly in the lack of health strategies and policies to address patient’s needs. The PARENTERAL study findings fill the current evidence gap by revealing the overall impact of SBS/CIF in Portugal, with a notable clinical, economic, and humanistic impact.
The clinical impact related to the PN support burden in Portugal was high, with only one PN free day per week. Although HPN was the major setting, non-negligible 13% of patients (n = 4) were treated almost exclusively in a hospital outpatient setting during the 12-month observation period.
One of the major study findings was the substantial HRU in SBS/CIF patients. SBS/CIF-related hospitalizations were notoriously frequent, with 77.4% of patients with at least one episode during the study period. An annual average per patient of two admissions and 31 days of LoS was estimated, which in practical terms translates into 1 month of hospitalization per year. This is consistent with the findings of a recently published retrospective cohort study in Danish SBS/CIF patients over a 46-year study period, which estimated an admission incidence of 2.5 episodes per year [27].
Catheter-related complications, mainly CRBSI, were responsible for over 80% of hospitalization episodes, which is in accordance with available literature evidence stating that CRBSIs remain to date the major limitation in long-term HPN, as a major source of morbidity and mortality in PN patients [10, 11]. Our study shows a significant economic impact associated with SBS/CIF management, with direct costs amounting to an overall annual average of EUR 65,000 per patient, namely, EUR 47,800 in adults and EUR 74,700 in children. PN support was the major cost-driver, and this difference may reflect different acquisition costs between commercialized and tailored PN used in the management of adult and pediatric patients, respectively. HRU was the second main cost-driver of which hospitalizations were the main source of financial burden, accounting for 46% of HRU costs in adults and 61% in children, followed by consultations with other healthcare professionals. These findings clearly show SBS/CIF burden in the NHS related to PN support complication management and SBS/CIF patient follow-up.
Although residual, it still should be mentioned that out-of-pocket costs are not refundable by the NHS, with patients having to support these costs many times required to manage the condition. In the children cohort, an annual average out-of-pocket cost of EUR 3,472.57 was estimated. Considering the last updated data (2014) regarding the average net annual household income (EUR 23.635) [28], out-of-pocket expenses in SBS/CIF pediatric management are estimated to account for over 15% of the average annual household income of a Portuguese family.
Evidence regarding economic impact of SBS/CIF management remains scarce. Results of a recent systematic literature review focused specifically on the costs of HPN program revealed the clinical and methodological heterogeneity between published studies which limits comparability with our findings [29].
A retrospective cost analysis performed by Canovai et al. [30] aimed to assess total annual costs in a cohort of stable and long-term intestinal failure adult patients, of which 59% diagnosed with SBS. With a median HPN duration of 5.3 years, costs stabilized by year five of PN support with an average annual cost per patient of EUR 68,278, with no significant variation between disease conditions [30]. In a prospective cohort study including nine Dutch children, with data collected since SBS/CIF diagnosis, the average annual cost of the patients who maintained PN with a follow-up ≥4 years was EUR 76.700, which is aligned with our findings [31].
QoL assessment in adult patients showed a clinically significant impaired physical component compared to the general population. Previous research in an SBS/CIF Swedish patient cohort revealed significant lower PCS when compared not only the population norm but also to patients with IBD. Although Crohn’s disease was the major primary cause of SBS in this cohort (85%), patient de-mographics and time on PN support were consistent with our population characteristics [14].
Considering the pediatric cohort, PedsQLTM average scores were slightly lower in comparison to the overall Portuguese norm. Deterioration was substantial in the school functioning domain, even when compared to children with other chronic diseases, reflecting the major impact of school absenteeism in SBS/CIF pediatric patients due to the high disease management burden for patients and caregivers. In fact, the impact of SBS/CIF in school functionality has also been previously reported elsewhere [32].
Particularly in children, the slight deterioration of QoL when compared to the Portuguese norm might be surprising due to the high burden that SBS/CIF imposes. However, this may reflect adjustment and coping mechanisms, a phenomenon well described in chronic patient populations [33].
To our knowledge, the PARENTERAL study is the first study conducted to characterize comprehensively SBS/CIF impact in Portugal, which translates into a major national investigation milestone. This study is indeed very important since there are no designated specialized centers on SBS/CIF management.
Although study sample is small, rarity of this disease on a global scale should be accounted. A recent multicentric national study aimed to determine the prevalence of pediatric CIF in Portugal, in which a total of 51 children/adolescents were identified, including 38 patients with SBS/CIF diagnosis [34]. As such, it is estimated that the current study included over half of the national SBS/CIF pediatric population.
Despite the strengths of this study, some limitations should be mentioned. First, the refusal to participate by two centers and the retrospective nature of the study design and chart review data collection. Due to the absence of centralized national patient registry, data validity in this retrospective analysis was dependent on accurate medical records.
Second, cost estimation focused solely on direct costs, not accounting for indirect costs associated with patient and caregiver productivity loss. It is reasonable to assume that these costs may be significant considering the debiltating nature of this condition and the pressure exerted on caregivers. Nevertheless, as mentioned by the authors of the previously mentioned SLR, no current study included productivity losses [29].
Lastly, although formal home care costs were not reported by patients, cost of informal caregiving may have a significant impact. At the time of study protocol development, no national decree regulated informal care, with the informal caregiver statute decreed only in 2019 [35] and subsidy conditions established in 2020 [36].
In conclusion, the PARENTERAL study revealed the wide-ranging impact of SBS/CIF in Portugal. The life-sustaining nature of PN support is offset by the high treatment burden and HRU associated with patient management and follow-up, with a notorious emphasis on incidence of admissions due to catheter-related complications, translating into high costs for the healthcare system and patient QoL deterioration. Although PN support remains the main standard-of-care in SBS/CIF, disease-modifying treatments have emerged and recently been made available in Portugal, which might avert the long-term impact of SBS/CIF.