Introduction
Ensuring the quality of gastrointestinal endoscopy is nowadays a priority for endoscopy units, endoscopists, and patients. In order to provide high-quality practice in gastrointestinal endoscopy, the European Society of Gastrointestinal Endoscopy (ESGE) has established the ESGE Quality Improvement Committee to analyze and define a list of performance measures (PM) in the different endoscopic areas [1-5].
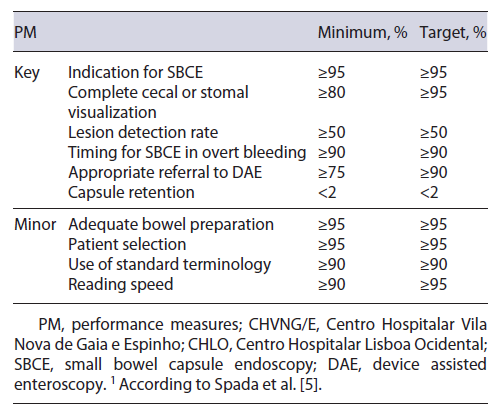
Recently, the ESGE Quality Improvement Committee developed a working group dedicated to the study of the small bowel (ESGE Quality Improvement Committee ES-BWG) that identified 10 quality PM (6 key and 4 minor), and for which a minimum and a target standard were established by consensus.
Rondonotti et al. [6] have conducted a survey for Italian endoscopic small bowel capsule endoscopy (SBCE) services that compared SBCE PM in their centers with ESGE standards [5]. The main goals were to provide a snapshot of the current clinical practice with SBCE and to identify areas that might be targeted in future programs. They concluded that only 4/10 (40%) SBCE procedural minimum standards were met by a relevant proportion of the centers (≥80%).
Likewise, quality in SBCE is still an issue to scrutinize. The authors aimed to evaluate and critically review the SBCE PM in 2 Portuguese centers with different SBCE platforms in an individual and global perspective. More-over, the authors aimed to explore if different SBCE platforms, Mirocam® (IntroMedic, Seoul, South Korea) or PillCam® (Medtronic, Yokneam, Israel), could impact some of SBCE PM results.
Materials and Methods
The authors conducted a cross-sectional analysis of consecutive SBCE performed from January 2018 to July 2019 in Centro Hospitalar Vila Nova de Gaia/Espinho (CHVNG/E), and from January 2017 to June 2019 in Centro Hospitalar Lisboa Ocidental (CHLO). The time period was selected to include approximately a similar number of SBCE procedures. The SBCE procedures were analyzed before the implementation of the ESGE PM for SBCE at the service level [5].
In both CHVNG/E and CHLO, SBCE were read or supervised by gastroenterology experts who have completed and reported more than 300 procedures. In CHVNG/E there was a mean of 7 operators (4 residents and 3 assistants) and in CHLO a mean of 3 operators (3 assistants). In CHVNG/E and CHLO, a medium of 3 SBCE and 2 SBCE were performed every week, respectively. In both centers, the patient’s medical history, indications, and con-traindications were previously assessed by a SBCE-dedicated gastroenterologist before the SBCE procedure.
According to previous statements [5, 7], patients considered to be at high risk of capsule retention were those with: known Crohn’s disease, symptoms of obstruction, long-term non-steroidal anti-inflammatory drugs (NSAIDs), abdominopelvic radiation and previous small bowel resection. ESGE recommends that a patency capsule should be offered to these patients before undergoing SBCE in order to reduce retention. As defined by ESGE technical review [7], SBCE retention was the identification of SBCE on abdominal radiological imaging over 14 days after ingestion. A “watch and wait” policy was instituted, as spontaneous passage of the capsule has been reported in the literature [8], and only 1 patient from CHLO required endoscopic removal by device-assisted enteroscopy (DAE).
In CHVNG/E, DAE is conducted in the same center. In CHLO, patients are referred to other centers to undergo DAE, since the procedure is not available in this center.
CHVNG/E: SBCE Protocol
SBCE were performed using the Mirocam® system following a protocol which consists of a clear liquid diet on the day before and an overnight fast with prior bowel preparation with 2 L of polyethylene glycol 12 h before the procedure. Real-time view 1 h after SBCE ingestion was performed to confirm SBCE presence in the small bowel, if the capsule remained in the stomach a prokinetic was administered, and if not effective after 30 min, endoscopic placement of SBCE in the duodenum was achieved. After confirming pylorus passage, patients resumed normal daily activities on an outpatient basis (except for hospitalized patients), ingested a light diet 4 h after, and the recorder was removed 12 h after SBCE ingestion or earlier if real-time viewing confirmed that the device has already reached the colon.
CHLO: SBCE protocol
SBCE were performed using the PillCam® system following a protocol which consists of a clear liquid diet on the day before with prior bowel preparation with a 2 L split-dose polyethylene glycol (PEG) regimen (1 L of PEG in the evening before and 1 L of PEG in the morning). The capsule was ingested with water and 40 mg of simethicone. Real-time view was performed 1 h after SBCE ingestion, ingestion of clear liquids 2 h after and a light diet 4 h after SBCE ingestion, and removal of the recorder at the end of the battery or once the capsule had been eliminated. If at real-time view capsule was still in the stomach, a similar protocol to CHVNG was followed to achieve capsule passage to the small bowel.
Data Collection
Data collection was performed by investigators from each center, in CHVNG/E this was executed by C.G. and in CHLO this was executed by C.O., A.R.F. and A.M. Data for each PM was collected and analyzed according to the description and the criteria suggested in the ESGE document [5]. The authors reported whether the ESGE standard (minimum and target) was met in each center (Table 1). The PM were also compared between the 2 centers, in order to evaluate if SBCE software or the center methodology could impact the results.
For the assessment of the rate of adequate bowel preparation, the authors considered an appropriate evaluation when reports described at least one of the Brotz scales (quantitative index, QI; qualitative evaluation, QE; or overall adequacy assessment, OAA) [9].
Statistical Analysis
Categorical variables were presented as frequencies and percentages, and continuous variables as mean and standard-deviation for parametric data and median and interquartile range (IQR) for non-parametric data. χ2 test or Fisher test, Student t test and Mann-Whitney U test were used to compare non-continuous and continuous data, respectively. For all comparisons, a p < 0.05 (2-sided statistical hypothesis test) was considered statistically significant. The statistical analysis was performed using the Statistical Package for Social Sciences (SPSS) program version 20 (IBM Cor-poration, Armonk, NY, USA).
Results
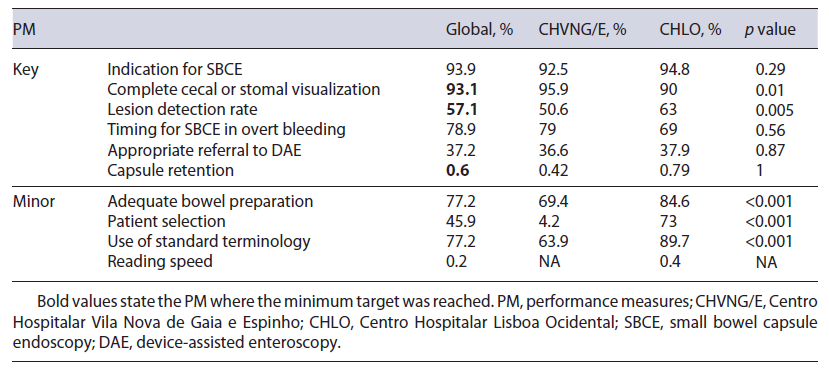
A total of 493 SBCE reports were audited (241 Mirocam® SBCE from CHVNG/E, and 252 PillCam® SBCE from CHLO), 53.3% were female and the mean age was 61 ± 18.4 years. The minimum standard was obtained in 3 out of 6 key PM (complete visualization, lesion detection rate, and capsule retention rate), and none of the 4 minor PM (Table 2).
Key Performance Measures
Indication for SBCE
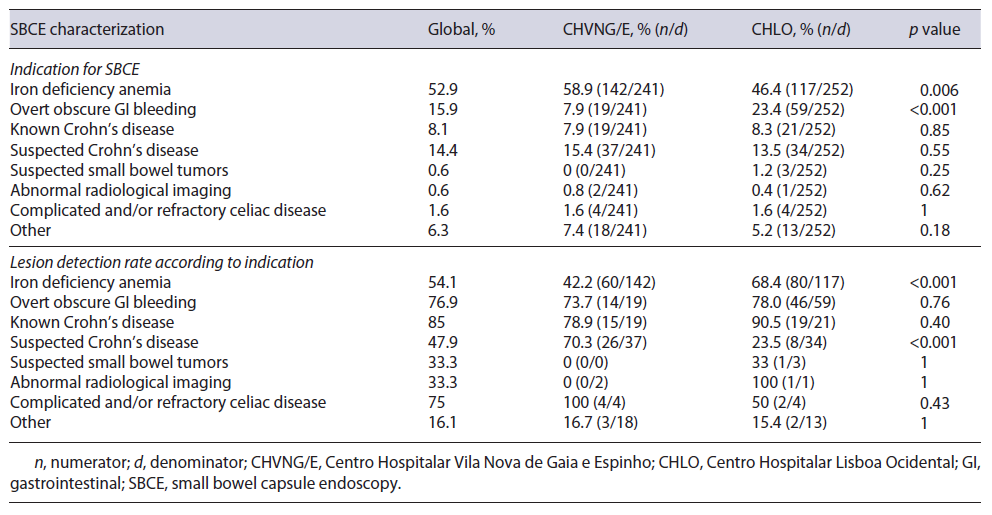
The use of a drop-down menu for indication has facilitated data acquisition for this PM. When evaluating the percentage of patients undergoing SBCE in accordance with the ESGE clinical guideline for SBCE [7, 10], 93.9% (92.5% CHVNG/E and 94.8% CHLO, p = 0.29) complied with this PM (Table 2). The characterization of SBCE performed at each center according to ESGE indications is stated in Table 3.
Complete Cecal or Stomal Visualization
The rate of SBCE reaching the cecum or stoma was 93.1% (n = 458, 231 CHVNG/E and 227 CHLO). This measure was documented in a written report including photo documentation. When comparing this rate be-tween centers, it was higher in CHVNG/E (CHVNG/E = 95.9% vs. CHLO = 90%, p = 0.01; Table 2).
Lesion Detection Rate
The ESGE document [5] specifies significant findings related to the indication as: P2 and P1 lesions according to the Saurin classification [11] for intestinal bleeding; ulceration, erosions, or strictures in the context of suspected/established Crohn’s disease; small bowel tumors and small bowel polyps. In our analysis, the global diagnostic yield (DY) was 57.1%. Although the DY was higher in CHLO (CHVNG/E = 50.6% vs. CHLO = 63%, p = 0.0056; Table 2), this was not reproducible within all indications, as in suspected Crohn’s disease the DY was higher in CHVNG/E (Table 3).
Timing of SBCE for Overt Bleeding
According to the ESGE working group [10], a SBCE should be performed within 14 days of the overt bleeding episode. The proportion of SBCE that complied with these recommendations was 78.9% (15/19 CHVNG/E and 41/59 CHLO, p = 0.56; Table 2). The medium time was 5 days (IQR 1.5-23; CHVNG/E: 2, IQR 1-9, and CHLO: 7, IQR 2-33.2, p = 0.09).
Appropriate Referral for DAE
The ESGE technical review [7] recommended that a DAE is indicated in patients with: significant findings at capsule endoscopy (P1 and P2 lesions according to the Saurin classification [11] for GI bleeding), suspicion of Crohn’s disease on SBCE (for biopsy), suspicion of a small bowel tumor (for biopsy and/or tattooing), when a submucosal mass is detected by SBCE and inherited polyposis syndromes when polypectomy is indicated. In line with these recommendations, 148 SBCE revealed pathological findings which may warrant further investigations. In this analysis, appropriate referral for DAE oc-curred in 37.2% (n = 55) [36.6% (30/82) CHVNG/E and 39% (25/66) CHLO, p = 0.87; Table 2]. Furthermore, 1 patient from CHLO was referred for DAE for management of SBCE retention (n = 1, 1 CHLO). Patients with indication for DAE who were not referred were: P1 or P2 lesions with controlled anemia and without persistent bleeding, or with a diffuse pattern (n = 47, 15 CHVNG/E and 32 CHLO), small bowel erosions or ulcerations with-out other significant lesions in patients with an uncertain diagnosis (n = 29, 27 CHVNG/E and 2 CHLO), non-ulcerated subepithelial lesions (n = 10, 6 CHVNG/E and 4 CHLO - in which 5 were suggested a CT enterography, 3 CHVNG/E and 2 CHLO), refractory celiac disease with-out malignancy suspicion (n = 3, 3 CHVNG/E), blue rubber bleb nevus syndrome (n = 1, 1 CHLO), suspicion of small bowel polyp referred for magnetic resonance imag-ing (n = 1, 1 CHLO), and suspicion of Meckel diverticulum referred for scintigraphy (n = 2, 1 CHVNG/E and 1 CHLO).
Capsule Retention Rate
Only 0.6% (n = 3, 1 CHVNG/E and 2 CHLO, p = 1; Table 2) of the patients had a SBCE retention.
Minor Performance Measures
Rate of Adequate Bowel Preparation
The rate of patients with an adequately prepared small bowel in SBCE according to a validated cleansing scale was 77.2% (370/479; CHVNG/E 69.4%, 161/232, and CHLO 84.6%, 209/247, p < 0.001; Table 2). For this measure, emergency SBCE or patients with active bleeding were excluded from the analysis (9 CHVNG/E and 4 CHLO).
Patient Selection
In total, 61 patients (24 CHVNG/E and 37 CHLO) were considered at high risk of capsule retention. From those, 45.9% (n = 28, CHVNG/E 4.2%, 1/24, and CHLO 73%, 27/37, p < 0.001) were offered a patency capsule (Table 2).
Use of Standard Terminology
The authors identified which SBCE reports followed the capsule endoscopy structured terminology (CEST) [12]. Overall, 77.2% (n = 380, 63.9% CHVNG/E and 89.7% CHLO, p < 0.001) complied with these recommendations (Table 2).
Reading Speed of SBCE
Only 1 SBCE report from CHLO stated an adequate reading speed of 10 frames per second.
Discussion
Our analysis of SBCE PM in 2 Portuguese centers revealed that the minimum standard was reached in 3 out of 6 key PM (complete visualization, lesion detection rate, and capsule retention rate), and none of the 4 minor PM. These data are similar to what was demonstrated by Rondonotti et al. [6], where 80% of the inquired centers reached 4 out of 6 key PM (adequate indication, complete visualization, lesion detection rate, and capsule retention rate) and none of the minor PM.
Adherence to appropriate indications for SBCE may optimize the use of limited resources (considering the high costs and time-consuming reading of SBCE) and protect patients from potential harms of unnecessary procedures [7, 10]. In our study, the minimum standard for this PM (indication for SBCE) was close to the target (93.9%). According to the study of Rondonotti et al. [6], 80.3% of the participant centers have achieved the minimum proposed standard (≥95%), mainly low-volume centers (<35 SBCE per year) when compared with medium- to high-volume centers (90.2 vs. 72.1%; p = 0.05). This may be due to less restriction on performing SBCE in centers with higher availability of this method, as our centers.
Complete small bowel visualization is a prerequisite for an adequate inspection of the mucosa, and incomplete examinations result in further costs due to the SBCE repetition and/or the need for an alternative investigation [5]. In this study, although the target standard was not reached, the minimum standard for completion rate was achieved (93.1%). The completion rate was higher in CHVNG/E (95.9% vs. 90%, p = 0.01), and this could be due to what was previously recognized by Choi et al. [13], stating that the longer reading time of the Mirocam® system may result in higher rates of complete small bowel examination (Mirocam® battery life 11-12 h and SB-3 ≥8 h). CHLO used a split-dose regimen and this did not appear to influence small bowel transit time [14]. It is uncertain if this split-dose regimen could impact the completion rate.
Lesion detection reflects adequate inspection of the small bowel mucosa, and both standards for this measure were accomplished (57.1%). Variations from expected rates raise the possibility of inadequate patient selection, procedure quality, reading, and/or reporting. In our study, we confirmed a suboptimal DY, around 16%, for indications apart from published recommendations which could ultimately compromise DY, as previously demonstrated in other studies (DY 7-23%) [15, 16]. Moreover, our DYs by indication were not inferior compared to previous literature [5], with rates that ranged between 31 and 68% for suspected GI bleeding, 6 and 38% for suspected Crohn’s disease, and 39% for active disease in known Crohn’s disease. In CHVNG/E, the overall DY was significantly lower compared to the DY of CHLO (50.6 vs. 63%, p = 0.006), although this was not seen in the DY for suspected Crohn’s disease, which was higher in CHVNG/E. According to society guidelines [17], the presence of at least 3 small bowel ulcers is highly suggestive of a diagnosis of Crohn’s disease, provided the patient has not been using NSAIDs for at least 1 month before the test. However, the results of SBCE have to be interpreted regarding other clinical bio-markers and imagiological parameters, to be able to be diagnostic. Apart from this subjective evaluation by the SBCE reader, the appreciation between small erosions and mucosal denudation may be difficult. As recognized by the small bowel working group [5], the DY could be affected by the reader interpretation of a relevant finding. Moreover, it is questionable if the difference in the DY could be associated to the SBCE platform, although previous data regarding the influence of SBCE platforms in the DY is somewhat contradictory [18, 19].
In the context of overt obscure GI bleeding, a timely SBCE was shown to increase the DY in various observational series [20-24], although a meta-regression model in a recent meta-analysis [25] revealed that timing of endoscopy was only significantly associated with the thera-peutic yield. In our analysis, the PM regarding the timing of SBCE in overt bleeding was not achieved, being 78.9%. Rondonotti et al. [7] also found that only around 30% of the Italian centers have reached the minimum standard. Nonetheless, this did not impact our DY for overt bleeding (76.9%), as this was superior to the most recent data (65.2%) [25]. Variations from expected targets may suggest suboptimal timing of procedures and different strategic approaches facing overt bleeding [5]. Rondonotti et al. [6] argued that patients with overt bleeding may be evaluated in clinical settings where the gastroenterologist is not immediately or routinely involved, and patients could only be referred to a gastroenterologist consultation once the acute event has resolved.
DAE is most often performed following a less invasive and simple procedure as SBCE. As stated by Rondonotti et al. [6], less than one third of the centers (32.2%) have reached the minimum standard of ≥75%, corroborating the difficulty to attain the referral target to DAE. In our study, the majority of lesions which did not motivate a subsequent DAE were P1 or P2 lesions with controlled anemia or without persistent bleeding (n = 47), small bowel erosions or ulcerations in patients with an uncertain diagnosis (n = 29) and non-ulcerated subepithelial lesions (n = 10). The authors consider that some SBCE-positive findings do not need a subsequent DAE, an invasive procedure, since some patients could be better managed with other treatment/diagnostic strategies, i.e., iron replacement therapy for some P1 or P2 lesions, or radiological methods for subepithelial lesions. The authors believe that the PM threshold concerning referral for DAE should be revised, as a significant proportion of patients can be managed by other treatment modalities (conservative, medical, surgical) as demonstrated by our series and Rondonotti’s study [6], as well as other published evidence [26, 27]. The referral rate from SBCE to DAE was similar between centers, and this was not affected by the DAE availability in CHVNG/E in relation to CHLO.
The optimal approach and timing of bowel preparation to enhance mucosal visibility in SBCE remains debatable, mainly its impact on completion rate and DY [7, 28, 29]. Although high performance standards are desirable, some PM for the rate of adequate small bowel cleansing do not appear to be attainable in clinical practice or even in clinical trials evaluating purgative solutions for small bowel cleansing [28-31]. In fact, it was neither attained in our study (77.2%) nor in Rondonotti’s study [6] (only 15.5% of participant centers reached ≥95%). CHLO have more often stated an adequate bowel preparation, and we hypothesized that this could be associated to the split-dose regimen as this was proved to impact the over-all and the distal assessment of small bowel cleansing [32]. Equally, the presence of multiple grading scales with diferent technical characteristics makes the classification with a validated scale more demanding and time consuming, which may explain why it was not stated in some of our reports.
As previously described, SBCE is generally a safe method with a low rate of capsule retention; however, certain underlying conditions and symptoms could predispose to capsule retention [32]. In our analysis, although only 45.9% of high-risk patients had performed a patency capsule, the capsule retention rate was low (0.6%), which implies appropriate patient selection in real-life scenarios. Likewise, in the Italian survey [6], only 10.9% of centers complied with this measure (≥95%). The use of a patency capsule is not consensual, as we found between our two centers (4.2% in CHVNG/E and 73% in CHLO, p < 0.001). The different adoption of patency capsule may be due to a lack of a standard protocol to detect patency capsule retention and to a non-negligible rate of false positives in abdominal radiography when identifying patency capsule location [33]. Moreover, it implies additional cost, it is not a risk-free procedure [34], and there are reported cases of SBCE retention after a negative patency capsule [35]. In contrast to previous data, a recent meta-analysis by Pasha et al. [36] showed that the retention rate in known Crohn’s disease was 4.6%, predominantly in patients with obstructive symptoms. In our sample, almost all Crohn’s disease patients did not present those symptoms, which may not have warranted a patency capsule. Furthermore, some patients with previous dedicated imaging modalities excluding signs of obstructive disease did not undergo a patency capsule.
There are no evidence-based recommendations regarding optimal frame rate for reading SBCE recordings. ESGE technical review [7] recommends a maximum speed of 10 frames per second in single view, considering that the reading rate should be slowed within the proximal small bowel where the risk of missing lesions appears to be higher. The reading speed is obviously platform dependent and the threshold of 10 frames per second is probably based on studies using the PillCam® SBCE platform and is certainly not equivalent to 10 frames per second in other platforms, such as Mirocam®. This could explain why this PM was not stated in our center’s reports.
The authors acknowledge some limitations of the study. Firstly, although data were collected on consecutively performed SBCE, the study has an observational design and retrospective data collection and analysis. Related to data collection, some parameters were difficult to check and may lack precision, such as ascertaining the factors associated with retention risk, evaluating the exact time between an overt bleeding episode and SBCE, some of which were not systematically included in the report and implied extensive searching of hospital databases. Secondly, parameters such as reading speed were not in-cluded in the report and could not be evaluated. Additionally, due to the descriptive nature of the study it could not provide an objective explanation for the observed variations between centers. Finally, the clinical outcomes were not evaluated and it was not possible to verify if accordance with the proposed SBCE PM impacts on rele-vant patients’ outcomes. In the author’s perspective this issue is of greatest importance and should be addressed in future studies.
Conclusion
Auditing PM is of utmost importance to improve quality of care. This study evaluates, compares between centers, and critically discusses the applicability in clinical practice of the expert consensus-based criteria and thresh-olds for SBCE PM. It may contribute to future revision of proposed PM, which should ideally be based on demonstrable patient’s clinical outcomes. It highlights some technical and organizational issues that could be ad-dressed for further quality improvement in SBCE but also raises questions on its applicability to different SBCE platforms. Furthermore, the platform-dependent partic-ularities should also be addressed in the construction of performance standards.