Introduction
Liver cancer is the fifth most common cancer and the second most frequent cause of cancer-related death, globally. Hepatocellular carcinoma (HCC) is the most com-mon form of liver cancer [1]. In Portugal, the number of hospital admissions for HCC has steadily increased in the last 20 years [2, 3] and the reported death rate is 4.3/100,000, which represents an increase of 66% between 2006 and 2012 [4].
Curative treatments such as radiofrequency ablation, surgical resection, and liver transplantation are recommended for early-stage HCC. Despite the active surveillance programs to detect early-stage HCC in patients with liver cirrhosis, a significant amount of them are still diagnosed in advanced stages. For these patients, treatment recommendations are transarterial chemoembolization and systemic therapy [1]. Sorafenib, an oral multikinase inhibitor, was the first effective systemic treatment in HCC and continues to be one of the standards of care for patients with well-preserved liver function (Child-Pugh A class) and with advanced tumors [5, 6]. There is no clear recommendation in Child-Pugh B patients, although cohort studies have reported a similar safety pro-file in patients of this class with no decompensation. Still, the reported outcome for Child-Pugh B patients from the non-interventional GIDEON trial was poor [1, 7].
In order to identify predictive factors of response to sorafenib treatment, a pre-planned subgroup analysis was carried out in the SHARP and AP trials [5, 6]. Similar results across subgroups (based on ECOG performance status, tumor burden, age, and hepatitis B virus infection) were observed in these studies, with sorafenib providing treatment survival benefit in all subgroups (although in the SHARP trial the benefit was less prominent in patients with extrahepatic spread; EHS). An exploratory analysis of the two studies showed that, although sorafenib benefit was observed in all subgroups, hepatitis C-positive patients, those without EHS, and those with a lower neutrophil-to-lymphocyte ratio (NLR) derived the most significant survival benefit [8]. Other studies have also described predictors of sorafenib benefit in observational studies of routine clinical practice [9-12], acknowledging the need to strengthen real-life evidence.
This study aimed to evaluate the survival and time to progression (TTP) with sorafenib therapy, as well as to identify associated factors for better survival and TTP, in a consecutive cohort of patients, in a single center, since the introduction of sorafenib in 2008.
Materials and Methods
Design, Setting, and Participants
We performed a retrospective cohort study including all adult patients (≥18 years) treated with sorafenib for HCC in a Liver Unit at Centro Hospitalar Trás-os-Montes Alto Douro (CHTMAD, Vila Real, Portugal) between January 2008 and December 2018.
All our patients who presented unresectable HCC, not eligible to locoregional therapies, and who had preserved liver function were treated with sorafenib. During the study period this was the only available systemic therapy. In our unit, the protocol for starting sorafenib consists of an initial dose of 200 mg twice daily, followed by a close monitoring of tolerance and a careful increase in dose up to a maximum 400 mg twice daily.
The inclusion criteria were the following: age ≥18 years, diagnosis of cirrhosis and HCC, initiation of treatment with sorafenib during the study period, follow-up in the CHTMAD Liver Unit (follow-up period until December 2018). The minimum follow-up considered for inclusion in the study was 6 months. The exclusion criteria were liver transplant and treatment duration inferior to 31 days (online suppl. Table 1; for all online suppl. material, see https://www.karger.com/doi/10.1159/000522572).
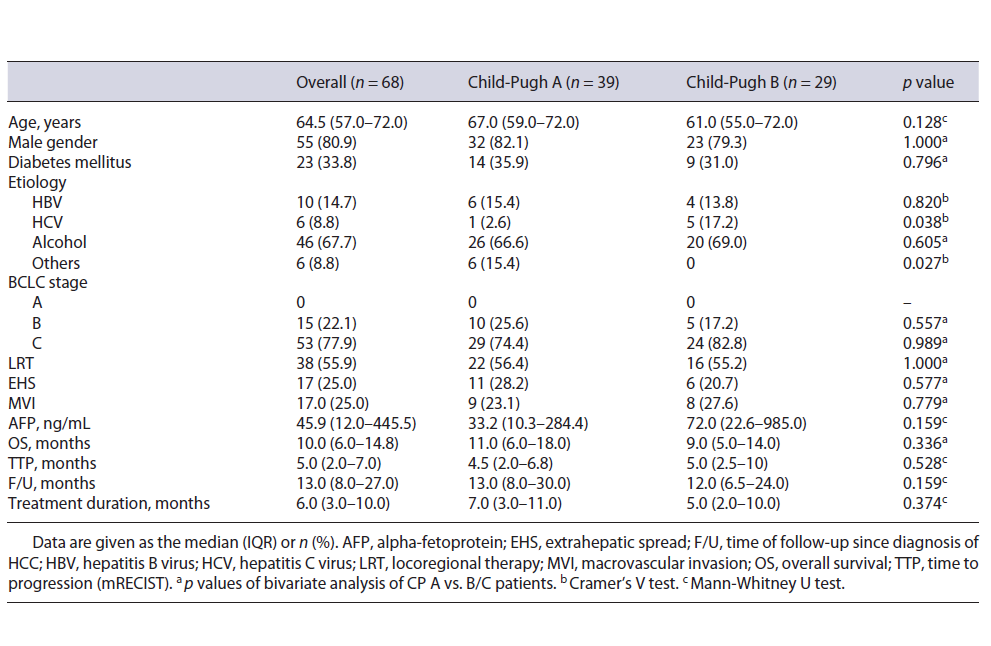
Table 1 Baseline characteristics of patients treated with sorafenib and comparison between groups according to Child-Pugh score status: univariate analysis
We made a sub-population analysis of the long-term survivors: patients surviving more than 12 months after initiating sorafenib were considered long-term survivors, since the estimated overall survival (OS) for patients undergoing sorafenib is around 10 months [5].
All therapeutical decisions related to HCC were discussed in a multidisciplinary meeting with several medical specialties (Hepatology, General Surgery, Medical Oncology, Interventional Radiology, and Radio-Oncology). Patients were followed with triphasic CT scan every 3 months (local protocol).
The current retrospective study was performed according to the requirements of the local ethics committee and complied with the Declaration of Helsinki principles [13]. Patient informed consent was not required for the current study, according to the guide-lines of the local ethics committee.
Data Collection and Endpoints
The patient selection was performed using the hospital pharmacy database. Data from patients prescribed with sorafenib for HCC in our Liver Unit from January 2008 to December 2018 were collected by reviewing their clinical records. The following data were collected at the start of treatment: demographics (birth date and gender); dates of diagnosis of HCC and start of treatment with sorafenib; the presence of diabetes mellitus; baseline liver disease (presence and etiology of cirrhosis, Child-Pugh score); laboratory results (alphafetoprotein, albumin, bilirubin, neutrophils and lymphocytes count); HCC characteristics (BCLC staging, size of hepatic lesions, extrahepatic metastasis, and macrovascular invasion; MVI), and previous treatment with surgery, chemoembolization, thermal or radiofrequency ablation. During the follow-up, the following data were collected: date and motive of sorafenib suspension, adverse effects, date of death, maximal tolerated dose, and TTP, evaluated by an experienced liver-related radiologist, using mRECIST [14]. Follow-up data were collected until July 31, 2019.
Patients were divided into two groups according to the Child-Pugh score (Child-Pugh A and B). The primary outcomes were survival and TTP of sorafenib-treated patients. The secondary aim was the identification of predictive factors of better survival and higher TTP.
Statistical Analysis
Baseline characteristics are described as number (%) for categorical variables and the median (interquartile range; IQR) for continuous variables (normal distribution was excluded by Kol-mogorov-Smirnov test). Associations of baseline characteristics with the primary and secondary endpoint were assessed using survival analysis with univariate and multivariate Cox regressions. Covariates in the multivariate models were selected if associated with a p < 0.05 in the univariate analysis. Survival of Child-Pugh groups was represented by a Kaplan-Meier survival plot. Statistical significance was set at p < 0.05. The statistical analysis was performed using SPSS Statistics® version 23.0 (IBM Corp., Armonk, NY, USA).
Results
Patients’ Baseline Characteristics
Sixty-eight patients were enrolled; 9 patients were excluded because of a treatment duration inferior to 31 days. The characteristics of the patients are reported in Table 1. Treatment was given in first-line therapy in 44.1% of the patients. The patients had a median age of 64.5 years and were mostly men. The etiology of cirrhosis was predominantly alcohol related (67.7%). Thirty-nine patients (57.4%) were classified as Child-Pugh A and 29 (42.6%) as Child-Pugh B. Among these Child-Pugh B patients, 14 had a score of 7; 9 had a score of 8, and 6 had a score of 9. There were no significant differences in BCLC staging and in the prevalence of previous locoregional therapy (LRT) between Child-Pugh A and B groups (Table 1). MVI was present in 25% of patients and 25% of subjects had other extrahepatic metastasis.
Table 2 Baseline characteristics of patients treated with sorafenib and comparison between groups according to Child-Pugh score status: univariate analysis
Twenty-three patients (35.4% of the total population) were considered long-term survivors. Among these, the median age was 67 years, 73.9% of patients were male, 78.3% had an alcohol-related cirrhosis, 56.5% were classified as Child-Pugh A, 73.9% were staged at BCLC C, 73.9% did not present extrahepatic spread, and 13.0% had portal vein thrombosis. Seven patients (30.4%) had a tumor size ≥5 cm. Only 4 patients (17.4%) presented an AFP >50 ng/mL. Ten patients (43.5%) tolerated 400 mg of sorafenib daily, against 21.7% that tolerated the maximum recommended dose of 800 mg daily. The median survival amongst this subgroup was 20 months (13-42). For the radiological response, evaluated by the modified RE-CIST criteria, none of the patients reached complete response, 13.6% presented a partial response, 46.2% had stable disease, and 40.2% underwent disease progression.
Survival
At the time of analysis (July 2019), 59 patients were dead (86.8%). The median duration of treatment of the alive patients was 15 months (IQR 10.0-20.5). The overall median survival was 10 months (IQR 6.0-14.8). The median survival of Child-Pugh A patients was 11 months (IQR 6.0-18.0) versus 9 months (IQR 5.0-14.0) in Child-Pugh B patients.
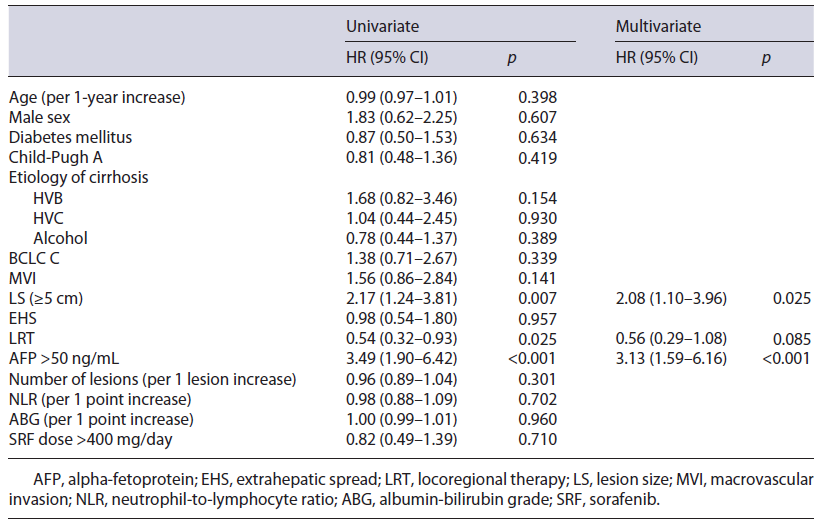
In univariate analysis, a larger lesion size (LS >5 cm), higher alpha-fetoprotein (AFP >50ng/mL), and no history of prior LRT were statistically associated with mortality (HR 2.17, 95% CI 1.24-3.81; HR 3.49, 95% CI 1.90-6.42; HR 0.54, 95% CI 0.32-0.93, respectively). EHS, NLR, albumin-bilirubin grade (ABG), sorafenib dose, and etiology of cirrhosis were not statistically associated with mortality, as presented in Table 2.
Multivariate analysis adjusted for age, gender, diabetes mellitus, Child-Pugh, and BCLC scores was performed. LS and AFP were shown to be independently associated with survival (LS: HR 2.08, 95% CI 1.10-3.96; AFP: HR 3.13, 95% CI 1.59-6.16).
Time to Progression
The overall median TTP was 5 months (IQR 2-7). The median TTP of Child-Pugh A patients was 4.5 months (IQR 4.5-6.8) and 5.0 (IQR 2.5-10.0) for Child-Pugh B patients.
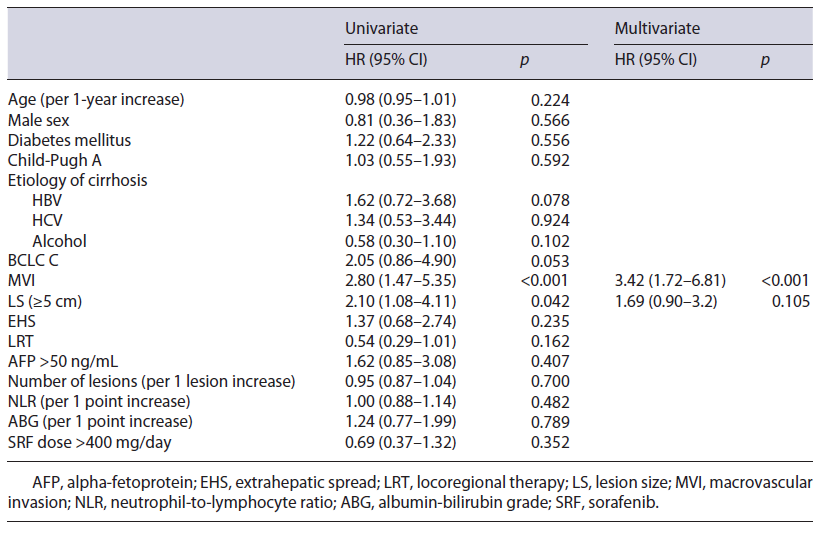
In univariate analysis, MVI and LS were statistically associated with TTP greater than 5 months (HR 2.80, 95% CI 1.47-5.35; HR 2.10, 95% CI 1.08-4.11, respectively). Multivariate analysis adjusted for age, gender, diabetes mellitus, Child-Pugh, and BCLC scores confirmed an independent predictive association of MVI with TTP shorter than 5 months (MVI: HR 3.42, 95% CI 1.72-6.81). LRT, EHS, AFP, NLR, ABG, sorafenib dose, BCLC stage, Child-Pugh score, and etiology of cirrhosis were not statistically associated with TTP, as presented in Table 3.
Table 3 Predictors of TTP shorter than 5 months in patients treated with sorafenib (n = 68): univariate and multivariate Cox regression analysis
Safety Data
Of the total patients treated with sorafenib, 52 patients (76.5%) reported at least one side effect (any grade). The most frequent adverse reactions were diarrhea (38.2%), anorexia (30.9%), fatigue (29.4%), skin reactions including hand-foot syndrome (17.6%), nausea (13.2%), vomiting (11.8%), arterial hypertension (2.9%) and dysphonia (1.5%). Among the total of patients with adverse events, 57.7% were Child-Pugh A patients and 42.3% were Child-Pugh B (p = 0.087).
Almost a third of the patients (27.9%) tolerated the maximum daily dose of 800 mg, while the remainder required dose adjustment due to any form of intolerance: 19.1% tolerated 600 mg daily and 47.1% tolerated 400 mg daily. Thirteen patients (19.1%) presented grade III-IV adverse effects leading to treatment discontinuation. Of the patients that tolerated the daily maximum dose of 800 mg, 77.8% were Child-Pugh A patients, and 22.2% were Child-Pugh B patients (p = 0.041).
Discussion
Sorafenib has been proven to be beneficial in selected patients with advanced HCC. However, few data are available on the use of sorafenib in a non-selected cir-rhotic population, namely on Child-Pugh B patients.
Hollebecque et al. [15] prospectively evaluated patients with advanced HCC treated with sorafenib and ob-served a higher survival among Child-Pugh A patients (11.1 months) compared with Child-Pugh B patients (4.5 months). Pressiani et al. [16] reported survival of 10 months in Child-Pugh A versus 3.8 months in Child-Pugh B patients, with similar adverse events in the two groups. Cardoso et al. [17] reported a very low OS of 6.8 months, with a median survival of 3.2 months for Child-Pugh B. Reis et al. [18], analyzing a subgroup of long survivors (>24 months under sorafenib), found that Child-Pugh A was an independent predictor of long-term survival, although also recognizing that sorafenib offers benefit regardless of baseline conditions or prognostic survival factors. The GIDEON trial, a global, non-interventional study, was conducted to evaluate sorafenib’s safety for HCC treatment under real-life practice conditions, particularly in Child-Pugh B patients. Shorter median survival was also observed in this group (4.8 months in Child-Pugh B vs. 10.3 months in Child-Pugh A patients), despite the safety profile favoring the use of sorafenib in Child-Pugh B patients [7]. The poor out-come of Child-Pugh B patients has also been demonstrated in other studies and has been attributed to the development of clinical features of liver insufficiency or tumor spread rather than safety profile issues [7, 8, 15].
In our sample, survival was not statistically different between the Child-Pugh groups, contrary to the previously described studies [5, 7, 15, 16]. TTP was also similar between the Child-Pugh groups. More recent real-life studies share similar results, showing no difference in survival between Child-Pugh A and B patients treated with sorafenib, but with significant heterogeneity amongst the population and OS [19, 20]. The incidence of adverse reactions in our population was similar to other studies, namely to what was reported in the SHARP study. The Child-Pugh class did not influence the incidence of adverse reactions, but Child-Pugh B patients seemed to tolerate less often the maximum sorafenib daily dose.
Alcohol-related cirrhosis was more prevalent in our population than in the previously described trials, although etiology was not associated with OS [7, 17, 20]. Since abstinence status was not confirmed, alcohol con-sumption could explain the worse liver function at enrolment in our study. Further experience and improvement in the management of sorafenib side effects have been associated with a longer treatment duration and better OS [21]. Of notice, treatment discontinuation was similar between Child-Pugh groups in our sample, which differs from other studies, such as in the GIDEON trial where Child-Pugh B patients had higher discontinuation rates (not drug related), and this might explain the similar survival between groups. Data related to liver function evolution and cirrhosis complications during the treatment period were not collected and so further rationale for these differences could not be enlightened.
Baseline characteristics and staging systems, including ECOG PS, Child-Pugh score, and BCLC stage, appeared to be prognostic factors for GIDEON survival, as were albumin, bilirubin, and ascites. Besides, measures of the extent of disease, including EHS, larger tumor size, a higher number of lesions, and AFP, were prognostic factors of shorter survival time [7]. Similarly, in a SHARP and AP trial subgroup analysis ECOG PS, albumin, bilirubin, larger target LS, a higher number of target lesions, and extent of disease factors (BCLC stage C, MVI, tumor burden, AFP, and high NLR) were identified as prognostic factors for poorer survival in patients receiving sorafenib [12]. In our analyses, primary LS larger than 5 cm, AFP higher than 50 ng/mL, and no history of LRT were significantly associated with higher mortality. However, only LS and AFP were independent predictors of mortality. MVI predicted a shorter TTP (5 months or less) in our sample.
Even though real-life observational studies provide an opportunity to assess treatment patterns in clinical practice in less selected patients, as an observational study, it is inherently limited by the lack of a randomized, controlled population and the potential for selection bias. Other limitations to our study include the size and heterogeneity of the cohort. Nevertheless, our analyzed prognostic factors are consistent with data from several studies about prognosis in HCC patients.
In the last few years, the reality of systemic treatment for advanced HCC is shifting, with new treatment modalities apporting more options, defying sorafenib as the standard of care.
Another first line option actually available is lenvatinib, a multikinase inhibitor that showed no inferiority when compared to sorafenib. Lastly, the recently approved combinations of bevacizumab with atezolizumab, or tremelimumab with durvalumab, showed superiority when compared to sorafenib, concerning OS and progression-free survival. However, a significant percentage of patients with advanced HCC may not be appropriate candidates for these options and still be considered for sorafenib or lenvatinib because of lower risk of serious bleeding, and also because of financial barriers [22].
Conclusion
In a northern Portuguese cohort of advanced HCC patients treated with sorafenib, there was no significant difference in survival or TTP in Child-Pugh A or Child-Pugh B patients treated with sorafenib as compared to more recent real-life studies. Lower primary LS and AFP were associated with a better outcome, and lower AFP was the main predictor of survival. It is important to mention that at the time of the analysis there was no other choice of treatment, after failure of LRT. Nowadays the reality is changing and new players are apporting more choices and results, but sorafenib remains a viable therapeutic option.















