Introduction
Neuroendocrine tumors (NET) are relatively uncommon gastrointestinal (GI) tract neoplasias, with a global annual age-adjusted incidence rate of 2/100,000 people per year [1]. However, the widespread use of endoscopy has led to increased detection of luminal GI, often at initial stages of disease. Not surprisingly, the majority of these initial stage GI-NETs are from the stomach, duodenum, and rectum, the most accessible areas to endoscopic exploration [2].
GI-NETs are classified as NET G1, NET G2 (both considered well-differentiated), and neuroendocrine carcinoma (NEC) G3 (poorly differentiated) based on the mitotic count and Ki-67 index [3]. Even though tumor grade is one of the most important prognostic factors (that is only correctly defined after resection), the size of the tumor is also an independent prognostic factor, increasing the risk of lymph node metastasis [4]. For this reason, most guidelines only recommend endoscopic resection (ER) as a treatment for small GI-NET, usually with less than 10-15 mm depending on the location, with every GI-NET larger than 2 cm being considered for surgery [4, 5].
Several studies and meta-analyses confirm the safety and effectiveness of ER for small GI-NETs. However, these studies include a small number of patients, and rarely long-term outcomes are reported [6-9]. Moreover, to our knowledge, no single study compared short- or long-term outcomes of the standard inject-and-cut endoscopic mucosal resection (sEMR) with more complex techniques such as EMR with a cap (EMRc) or endoscopic submucosal dissection (ESD).
In this retrospective study, we analyze long-term out-comes after ER of GI-NETs in the stomach, duodenum, and rectum. Moreover, we compare the short and long-term outcomes of the different ER methods.
Materials and Methods
Patients and Lesions
A retrospective observational study was performed. Pathological database of the Portuguese Oncology Institute of Porto was searched for GI-NETs diagnosed between 2010 and 2020. After evaluation of the pathological report, patients with non-GI NET, pancreatic, small bowel (with the exception of duodenum), appendix, or colonic (with the exception of rectum) NET were excluded from the analysis. The clinical records of all the other patients were analyzed. At this stage, additional exclusion criteria were non-endoscopic initial treatment (surgery or somatostatin analogs), GI-NET only present in biopsies, endoscopic diagnosis/treatment only with cold or hot-snare resection (without submucosal injection), endoscopic treatment at other hospital, or less than 12-month follow-up. At the end, only patients with GI-NET from stomach, duodenum, or rectum treated in our institute by sEMR, EMRc, or ESD with at least 1 year of follow-up were included in the analysis. In Figure 1, we can see the flowchart for patient enrolment.
ER Procedures
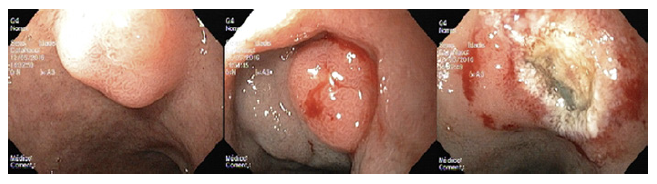
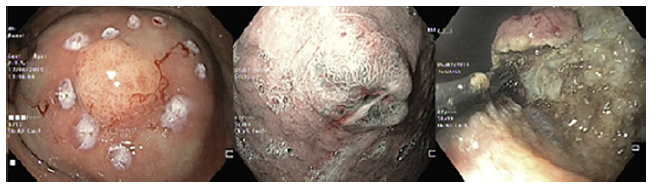
Standard EMR (EMRs) was defined as the conventional tech-nique of tumor resection with hot snare technique after submucosal injection with normal saline and diluted adrenaline (1:10,000 to 1:50,000 dilution) (Fig. 2). EMRc was performed with a transparent cap (Olympus, reusable oblique cap) at the tip of conventional upper GI endoscope and a crescent-type snare (EMR snare, Olympus). Hotsnare resection was done after submucosal injec-tion with normal saline and diluted adrenaline (1:10,000 to 1:50,000 dilution) and suction of the lesion into the cap. ESD was performed as previously described (Fig. 3). Briefly, small coagulation marks were made around the lesion and then submucosal injection was performed with saline, diluted epinephrine (1:50-100,000), and methylene blue. After elevation, 3-4 incisions were made with a needle knife (Olympus®) to get access to the submucosal layer, and an insulated-tip knife (mainly IT-KnifeTM; Olympus®) was used to perform circumferential dissection using the Endo Cut mode (Olympus electrosurgical unit, 80/60 W). Complete dissection was then performed in the Endo Cut or swift coagulation mode, with additional submucosal injection whenever necessary. The procedures were performed mainly under general anesthesia (with orotracheal intubation); deep sedation was restricted to a minority of procedures.
Definitions and Follow-Up
En bloc ER was defined as ER in one single fragment versus piecemeal resection defined as two or more fragments ER. Complete ER was defined as no evidence of macroscopic disease after ER, independent of the type of ER (en bloc or piecemeal). Adverse events were defined as immediate (during procedure) or delayed complications (not apparent during the procedure). Bleeding as a complication was defined as intraprocedural bleeding requiring non-planned hemostasis (immediate bleeding) or as melena or hematochezia after the procedure (delayed bleeding), independently if additional interventions were required or not. Perforation was defined as a bowel wall penetration identified during the procedure (immediate perforation) or as symptoms compatible with perforation with imagiological (CT) confirmation of that (delayed perforation). Endoscopic size was defined as the estimated macroscopic size attributed by the gastroenterologist in the endoscopy report.
All the histological findings were evaluated by two pathologists, with at least one of them being experienced in GI-NET evaluation, with each specimen being graded according to the WHO classification [3]. Histological complete resection was defined as margins free of tumor, both lateral and vertical margins. Histological maximum size of the lesion was considered as the maximum diameter from one side to the other.
All patients were followed-up with periodic endoscopy (at least one per year), serum chromogranin A (at least one per year) and imagiological methods, generally PET-CT with somatostatin receptors markers (as needed). Local recurrence was defined as histologically confirmed diseased at the site of ER, and systemic recurrence as histologically confirmed ganglion, liver and/or another organ NET metastasis.
Statistical Analyses
Data were expressed as mean + standard deviation or as median and interquartile range (according to the dispersion) for continuous variables and as frequencies and/or proportions for categorical variables. Differences in outcomes were compared using independent t tests for numerical variables and χ2 tests for categorical variables (p values were considered significant if they were <0.05). Multivariable logistic regression model was constructed to identify risk factors for systemic recurrence (including age, sex and variables with p < 0.20 at univariable analysis). All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) software (version 27.0; IBM Corp., Armonk, NY, USA).
Results
Patient and Lesion Baseline Characteristics
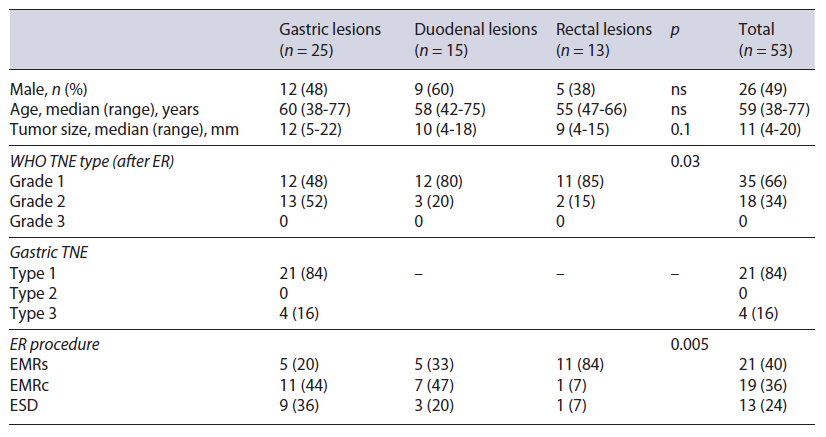
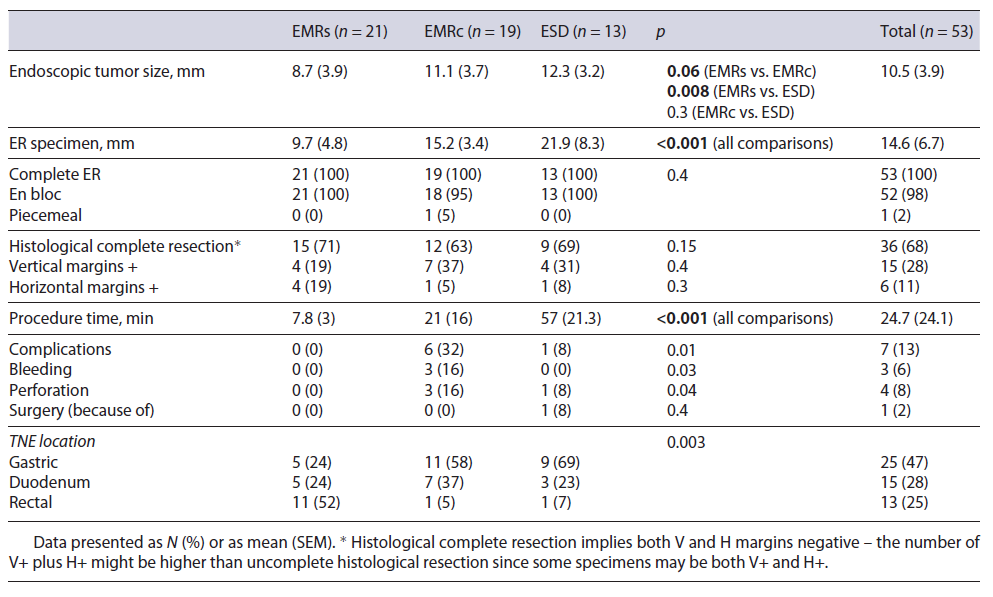
A total of 53 patients were included in the analysis (Table 1). The median age was 58 years old and 49% were male, with no differences between the groups. The median size of the lesions was 11 mm (4 minimum, 20 max-imum) with a non-significant trend for larger lesions in the stomach group. Only NETs grade 1 on biopsies were considered for ER (with a histological upgrade in the resection specimen to grade 2 lesions in 34% of the lesions). Twenty per cent of gastric NETs were type 3 and both this feature and size translated into more advanced lesions in the stomach group (52% grade 2 lesions vs. 20% and 15% in the duodenum and rectum, respectively, p = 0.003). Endoscopic ultrasound (EUS) was performed in 51% of the patients, generally for lesions bigger than 10 mm.
Clinical Outcomes
The clinical outcomes of the different ER methods are summarized in Table 2. In general, EMRs was used for smaller lesions. There were no differences between the techniques regarding complete endoscopic and histological resection, with only one lesion being resected in piecemeal in the EMRc group. Even though complete ER was always achieved, histological complete resection rate was only of 68% (no differences between the groups). ESD was significantly longer than EMRc and EMRc significantly longer than EMRs (57, 21, and 8 min, respectively, p < 0.001). The complication rate was significantly higher in the EMRc group (2 duodenal and 1 gastric perforation) when compared to the other two groups (EMRc 32%, ESD 8% and EMRs 0%, p = 0.01). However, surgery because of complication was only needed in one patient, after duodenal ESD.
Follow-Up
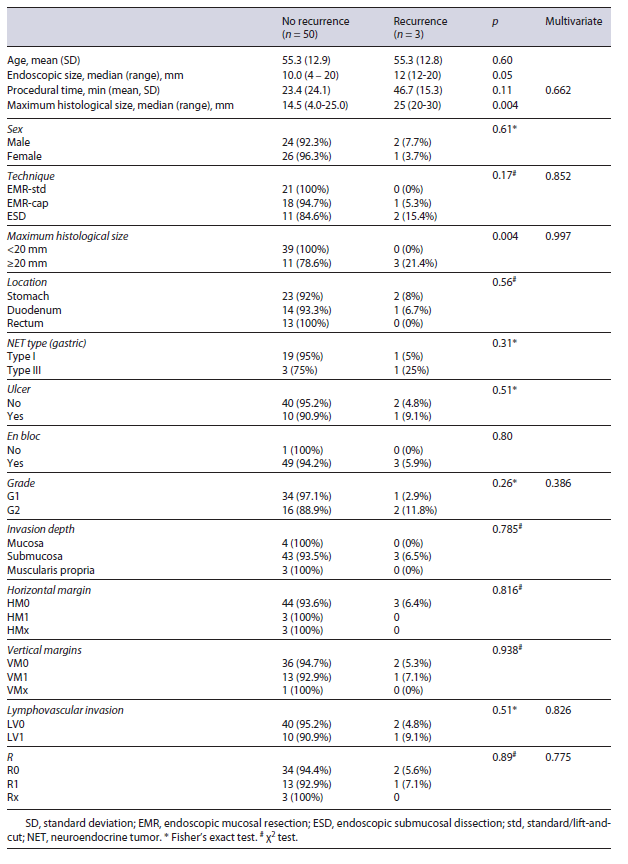
The mean follow-up was 44.6 months (range 12-102 months, no differences between the ER groups), and in this period there was only one local recurrence (2%), which was treated by another ER. There were 6 new lesions identified and treated by ER, all type 1 lesions in the stomach. Systemic recurrence occurred in 3 patients (1 only nodal and 2 liver and nodal disease, one of this with carcinoid syndrome), one case of type 1 gastric, other case type 3 gastric, and one duodenal NET. The mean time between diagnosis and systemic recurrence was 9 months (range 6-12 months). Only the duodenal NET patient with systemic recurrence died because of NET (after surgical treatment). Three additional patients died during follow-up due to NET-unrelated causes (specific disease-free survival 98%, global survival 92%).
Risk Factors for Recurrence
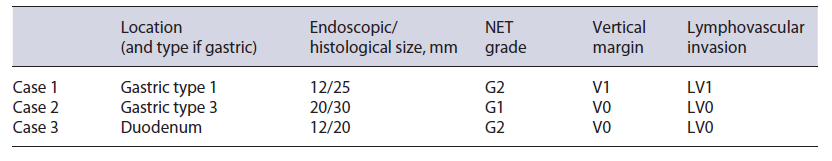
There was only one local recurrence, a 4-mm recurrence 3 years after R1 resection of type 1 gastric TNE that was treated effectively by hot snare technique. There was no statistically significant risk factor for local recurrence. Only one out of 17 (6%) R1 resections locally recurred.
Distant recurrence occurred in 3 patients (Table 3). The only identified risk factors for distant recurrence (Table 4) were the ones related to the size of lesion. Median endoscopic size of the lesions that recurred was 12 mm (p = 0.05) with all the recurrent lesions having a maximum histological size larger or equal to 20 mm (p = 0.004). Histological maximum size was the strongest risk factor for distant recurrence (p = 0.004).
For metachronous lesions, the only risk factor was type 1 gastric NET (p < 0.001).
Discussion
GI-NETs are being more frequently diagnosed and treated by ER methods. Even though several studies show the effectiveness and safety of different ER methods for the treatment of GI NETs, long-term outcomes are rarely described. To our knowledge this is the first study that focuses on long-term outcomes after several ER methods for the treatment of luminal GI NETs. Our results confirm that ER should be a first-line therapy for small GI NETs providing curability in most cases.
There are some limitations to our study. First, we have a relatively small sample size of 53 patients. Secondly, by including all the organs we should be careful to interpret and generalize our results. Thirdly, even though similar, follow-up was not standardized between patients and so recurrence data should be interpreted with caution. Fourthly, band-EMR was not applied in any case, and so no conclusion can be made about this technique. Finally, the retrospective nature of the study should limit our conclusions regarding comparison of the several ER methods since selection bias is highly likely. Nevertheless, our study has several strengths. To our knowledge, it is the first study to focus on long-term outcomes after ER of luminal GI-NETs. We show that, independently of the organ, ER is a safe and highly effective therapy for small luminal GI-NETs. Moreover, we provide comparative data of 3 different ER methods that were rarely addressed in the literature. Our results show that EMRc should be avoided for the treatment of GI-NET since the risk of complications appears too high to justify this technique. Even though most complications can be handled endoscopically, they prolong hospitalization of our patients with greater costs, and if safer techniques are available, they should be preferred. Our results also demonstrate that, independently of the technique and margins, if ER is complete, local and distant recurrence is highly unlikely and does not seem to affect the global prognosis of these patients.
In a perorgan analysis for gastric NET, there are only few comparative studies of ER methods, all of them with a limited number of cases. Kim et al. [10] compared EMR and ESD in type I g-NETs and showed a higher complete resection and higher complication rate for ESD (both non-significant). Based on this, they concluded that ESD might be a better option for the treatment of gastric NET. However, no clinical advantage was seen in this study. In fact, other studies found no tumor recurrence after ER (EMR and ESD) during the follow-up of gastric NET G1/G2, even in patients with positive margins [11, 12]. This is in accordance with our results that showed that the importance of positive margins after complete ER regarding clinical and long-term outcomes is probably minimal since there was only one local recurrence after R1 resection (6% risk), and a small easily to treat recurrence. Regarding type 3 (sporadic) gastric NETs most guidelines still consider surgery as the best approach [3, 4]. However, Kwon et al. [13] suggested that ER can be safely performed in type 3 gastric NETs if <20 mm, G1 grading, confined to SM, and without lymphovascular invasion. In accordance, we were able to treat efficaciously 3 out of 4 type 3 gastric NET, with the only type 3 tumor that recurred systemically being a >15-mm tumor (the other 3 were 10- to 13-mm lesions). Even though these results should be interpreted cautiously, these 2 studies suggest that at least for <15-mm type 3 lesions, ER (particularly by ESD) may be a safe option.
For duodenal NETs, there are only some series, and they all include a small number of patients and a short long-term follow-up. Even though complete resection rates are high for both EMR and ESD, ESD perforation rates in the duodenum appear exceedingly high (>20%) [14-16]. In fact, our ESD duodenal perforation rate in our study was 1 in 3 (33%), and we now favor EMR-based techniques for duodenal NET ER.
For ER of rectal NET, the number of studies is considerable with evidence gathered in some meta-analyses (even though substantiated mostly on single-center studies with small groups of patients) [7, 8]. Based on significantly higher complete pathological rates both with modified EMR techniques and ESD compared to sEMR, with a similar safety profile, the authors concluded that modified EMR techniques and ESD should be preferred over sEMR. Despite this conclusion, long-term clinical out-comes were not different between the groups, with local recurrences being exceedingly rare (0.89%) even after incomplete pathological resection [7]. In fact, in our study most rectal NETs were treated by sEMR, and despite only 70% complete pathological resection rate, all patients were cured with no long-time local or systemic recurrence.
Taking all together, regarding short-term outcomes, all ER methods were highly efficacious in treating small luminal GI-NETs. Even though we did not find higher complete pathological rates, EMRc and ESD were select-ed for bigger and depressed lesions, particularly ESD, as they are associated with a significantly bigger specimen size. EMRc was associated with a significantly higher complication rate and, in our opinion, should be avoided in the treatment of luminal GI-NET. So, for most gastric, duodenal, and rectal lesions <10-12 mm in size, sEMR probably should be favored over ESD if lesion characteristics suggest that en bloc complete ER is feasible. If the lesion size is >12 mm or if the lesion shows depressed morphology, then ESD, even though more cumbersome, should be preferred over sEMR at least in the stomach and rectum, since in the duodenum the high perforation rates make it prohibitive.
Regarding long-term outcomes, our study suggests high curative rates after successful ER of small luminal GI-NETs. Local or systemic recurrences are an exception even after R1 resections. Thus, in our opinion, positive margins after a complete ER should not guide further treatments or significantly influence further management. However, lesion size >12 mm significantly increases the risk of systemic recurrence. So, in these cases, before considering ER, multidisciplinary evaluation is advised. Nevertheless, even for these lesions, ER should be an option, particularly if the location of the tumor may imply a more aggressive surgery and/or when the patient is not fit for surgery. Furthermore, since maximum histological size is the strongest risk factor for recurrence, if after ER maximum histological size is at least 20 mm, consideration should be given to additional treatments in a multidisciplinary discussion. If ER is decided for these lesions, frequent (annual/bi-annual or as clinical needed) imagiological (e.g., PET-CT) follow-up is advised since the risk of systemic recurrence is high. Regarding endoscopic surveillance, our results suggest that besides type 1 gastric NETs, there is no need for a strict endoscopic follow-up, since local recurrence or new lesions are exceedingly rare. We recommend endoscopy 1 year after ER and, if there is no evidence of local recurrence, there is probably no need for further endoscopic surveillance (if positive margins are present, endoscopy 3-5 years after resection might be considered).
In conclusion, ER is a safe and highly effective treatment particularly for <12-mm luminal GI-NETs and when the maximum histological size post-ER is <20 mm. sEMR is an easy and safe technique that is associated with long-term curability, even if there are positive margins, and it is probably the best therapeutic option for most luminal GI-NETs. ESD appears to be the best option for le-sions that cannot be removed en bloc with sEMR. Multicenter, prospective randomized trials evaluating long-term outcomes should confirm these results before strict recommendations can be made.