Introduction
Transarterial chemoembolization (TACE) is the first-line treatment for patients with non-resectable hepatocellular carcinoma (HCC) in the absence of decompensated cirrhosis, large tumor size (>10 cm), comorbidities, portal vein thrombosis, or extrahepatic spread [1-3]. The survival benefit of TACE in patients with intermediate-stage HCC is well established [4]. However, this therapy frequently loses its therapeutic efficacy over time, despite repeated procedures, leading to TACE refractoriness [5].
For patients without an adequate response after TACE, the concept of therapeutic stage migration can be considered [6]. Systemic therapy (ST) has been demonstrated to be effective in patients after TACE failure [7]. Furthermore, recent studies suggest that, in patients with HCC and TACE refractoriness, ST might improve the prognosis, when compared with repeated TACE procedures [8-10]. Moreover, several studies demonstrate a benefit of systemic treatment as an adjuvant to TACE [11]. As such, ST can be considered in patients with intermediate-stage HCC [1, 7]. However, ST, similar to locoregional therapy, requires preserved liver function, and patients that develop early deterioration of liver function following TACE are deprived of ST as a therapeutic option [12]. To complicate matters, up to 25% of patients with TACE refractoriness develop Child-Pugh B/C after initial TACE [8, 9]. Furthermore, it has previously been described that repeated TACE procedures are associated with deterioration of hepatic reserve, even though recent studies might challenge the relevance of this association [11, 13]. Defining predictors of early liver decompensation in patients with HCC proposed to TACE could potentially help in better treatment selec-tion in such patients. Previous works fail to propose predictors of early deterioration of liver function before TACE refractoriness.
This study aims to evaluate possible predictors of over-all and 6-month survival free of liver decompensation before TACE refractoriness in a cohort of Western patients with intermediate-stage HCC submitted to TACE.
Material and Methods
Study Design
We performed a retrospective study of all consecutive patients with intermediate-stage HCC submitted to drug-eluting polyvinyl alcohol microspheres TACE (DEM-TACE), as proposed by a multidisciplinary board, in a tertiary center between January 2010 and October 2020. This study was carried out in compliance with the ethical principles outlined in the Declaration of Helsinki and was approved by the Ethical Committee of our center. The requirement for written informed consent was waived. Inclusion criteria where: (i) radiological or histological evidence of HCC in accordance with the diagnosis criteria of American Association for the Study of Liver Diseases guidelines, (ii) intermediate-stage HCC in accordance with Barcelona Clinic Liver Cancer staging system, (iii) Child-Pugh class A cirrhosis before DEM-TACE, and (iii) treatment with TACE. Exclusion criteria were: (i) comorbidities of other serious diseases (American Society of Anesthesiologists physical status >2), (ii) lack of appropriate follow-up (clinical evaluation or radiological tumor evaluation within 3 months after initial DEM-TACE), (iii) other treatment previous or concomitant with TACE, and (iv) treatment with conventional TACE [14]. All patients were submitted to a biochemical and medical evaluation before initial TACE.
Transarterial Chemoembolization Procedure and Subsequent Follow-Up
After catheter insertion in the celiac artery, guided by angiography, a microcatheter injects contrast into the common hepatic artery, identifying the arteries that feed the tumor. The microcatheter is then advanced as far as possible in the segmental or subsegmental branches feeding the tumor. Drug-eluting microspheres loaded with doxorubicin are injected into the tumor’s supply arteries until blood flow is obstructed. The selection of doxorubicin dose is determined by our institution’s protocol.
Objective response rate (ORR) to TACE was evaluated 1 month after each procedure with magnetic resonance or computer tomography scan, in accordance with modified response evaluation criteria in solid tumors (mRECIST) [15]. Subsequently, every 3 months imaging was performed and intrahepatic recurrences or presence of viable tumors dictated additional treatment, TACE, or other indicated treatment depending on TACE refractoriness criteria.
Adverse events were accessed retrospectively in patient records and were classified according to Common Terminology Criteria of Adverse Events (CTCAE) 5.0 [16]. Only CTCAE grade ≥3 adverse events were recorded.
Definition of TACE Responder, Liver Decompensation, and TACE Refractoriness
ORR was defined as (i) present in patients with complete response or partial response and as (ii) absent in patients with stable disease or progressive disease in accordance with mRECIST criteria. Liver decompensation was defined as irreversible exacerbation from Child-Pugh class A to Child-Pugh class B or C after first TACE. TACE refractoriness, in accordance with the Japan Society of Hepatology, was defined as (i) ≥2 TACE procedures with each reevaluation showing progression in tumor number or insufficient response of the treated tumors (viable lesions >50%) or (ii) appearance of extrahepatic spread or vascular invasion [17].
Outcomes
The primary outcome was overall survival free of liver decompensation, defined as the time interval from the first TACE to liver decompensation. Secondary outcomes were 6-month survival free of liver decompensation, overall survival free of TACE refractoriness (time interval from the first TACE to TACE refractoriness), and overall survival (time from the first TACE to death).
Statistical Analysis
All variables were presented as categorical variables, with counts and percentages. Overall survival free of liver decompensation was defined as the time interval between initial TACE and development of liver decompensation. Patients who survived without liver decompensation at the last follow-up date (December 10, 2020) or were lost to follow-up were censored. Patients with TACE refractoriness before liver decompensation were censored at the time of TACE refractoriness. Overall survival free of liver decompensation rates were estimated using the Kaplan-Meier method and compared using the logrank test. Multivariable analysis, using Cox-regression, was performed for the analysis of the following out-comes: overall and 6-month survival free of liver decompensation, overall survival free of TACE refractoriness, and overall survival. All variables statistically significant in the previous univariable analysis were included in this analysis. Multiple imputations had been performed with 5 independent draws for missing values. The interactions between predictors were also tested.
Possible models for risk stratification of overall survival free of liver decompensation were then developed based on the above analyses and performance was measured by likelihood ratio chi-square. A ROC curve analysis was developed to determine the best cut-off value for the model. Kaplan-Meier analysis compared overall survival free of liver decompensation between the two groups created.
Differences were considered statistically significant when corresponding p values were less than 0.05. All statistical analyses were performed using SPSS Version 23 (IBM Corporation, Chicago, IL, USA).
Results
Patients’ Baseline Characteristics
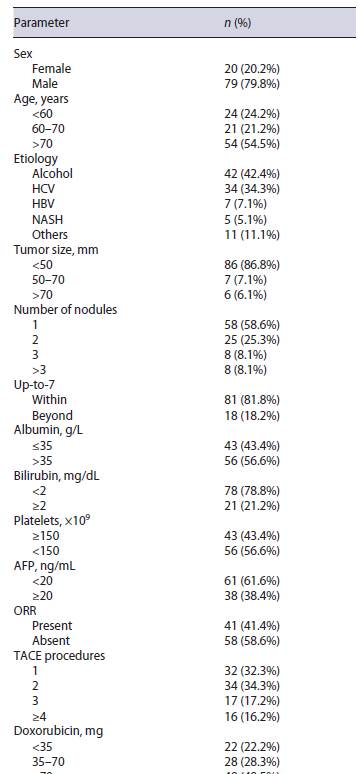
From a total of 161 patients with intermediate-stage HCC and Child-Pugh A cirrhosis treated with TACE, 99 patients were included (Fig. 1). Mean age was 73 (IQR 59-86) years, 78 (79%) were male. Median follow-up time was 40 (IQR 11-62) months. The etiology of hepatic disease was alcohol in 42%, chronic hepatitis C in 34%, chronic hepatitis B (HBV) in 7%, non-alcoholic steato-hepatitis in 5%, and others in 11% (Table 1). Median tumor size was 37 mm (10-180 mm). Median tumor number of nodules was 1.6 (IQR 1-2). Eighty-one patients (82%) were within up-to-7 criteria and 18 patients (18%) were beyond up-to-7 criteria. Median albumin was 35.5 g/L (IQR 34-38), median bilirubin was 1 mg/dL (IQR 0.7-1.5), median alpha-fetoprotein was 10.9 ng/mL (IQR 4-70), and median international normalized ratio was 1.2 (IQR 1.1-1.3). No patient presented ascites or hepatic encephalopathy.
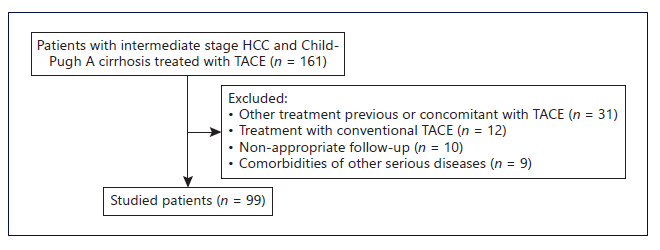
Fig. 1 Flowchart regarding patient selection. HCC, hepatocellular carcinoma; TACE, transarterial chemoembolization
Overall and 6-Month Survival Free of Liver Decompensation before TACE Refractoriness
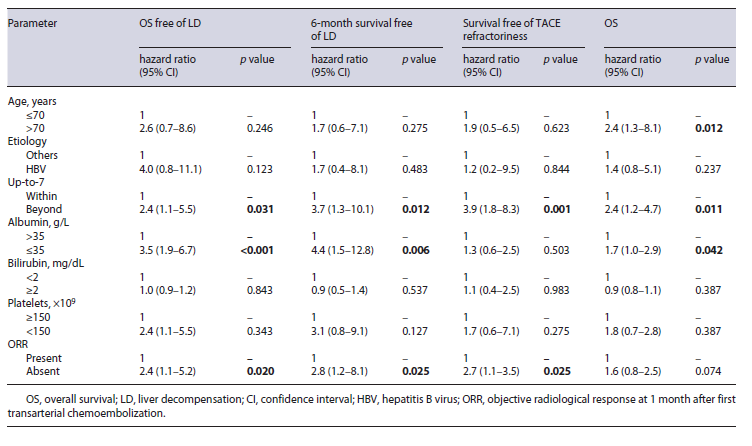
Liver function deteriorated to Child-Pugh B/C before TACE refractoriness in 51 (51.5%) patients and median time to liver decompensation was 14 (IQR 8-20) months, after first TACE. In univariable analysis, overall survival free of liver decompensation was significantly longer in patients within versus beyond up-to-7 criteria (median, 40.9 vs. 20.3, p = 0.041), albumin >35 versus ≤35 mg/dL (median, 43.7 vs. 24.5 months, p < 0.001), bilirubin <2 versus ≥2 mg/dL (median, 43.9 vs. 18.9 months, p = 0.029), and presence versus absence of ORR (median, 56.7 vs. 27.6 months, p = 0.002) (Table 2). Inmultivariable analysis, beyond up-to-7 criteria (HR 2.4, p = 0.031), albumin <35 mg/dL (HR 3.5, p < 0.001), and absence of ORR (HR 2.4, p = 0.020) presented a negative association with overall survival free of liver decompensation (Table 3). Moreover, 6-month survival free of liver decompensation presented a negative association, in multivariable analysis, with beyond up-to-7 criteria (HR 3.7, p = 0.012), albumin <35 mg/dL (HR 4.4, p = 0.006), and absence of ORR (HR 2.6, p = 0.025) (Table 2).
Table 2 Univariable analysis for overall survival free of liver decompensation, 6-month survival free of liver decompensation, survival free of TACE refractoriness, and overall survival
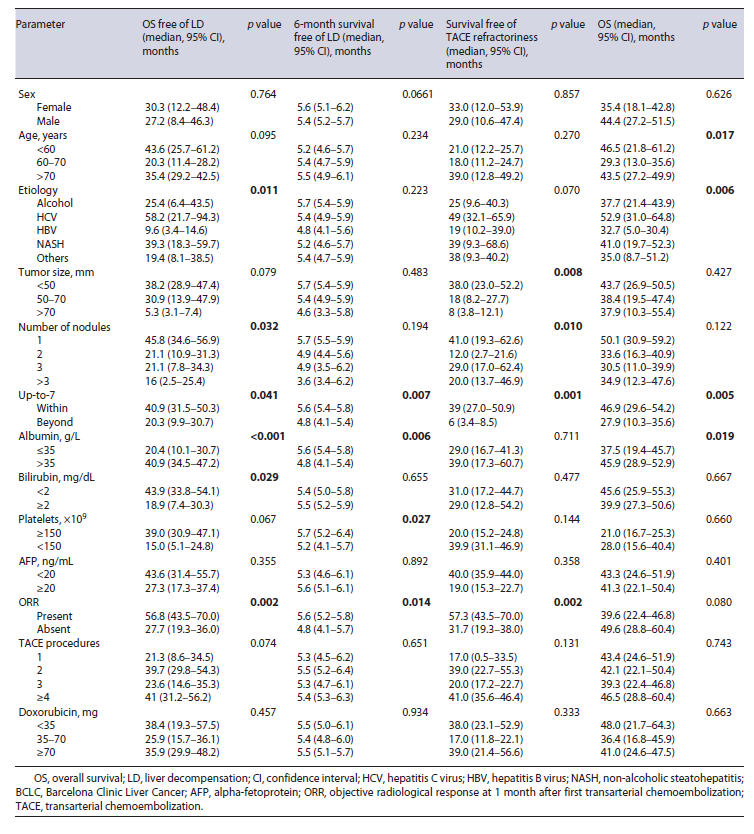
Table 3 Multivariable analysis for overall survival free of liver decompensation, 6-month survival free of liver function deterioration, survival free of TACE refractoriness, and overall survival
Using up-to-7-criteria, albumin, and ORR, we created a model predictive of 6-month survival free liver decompensation. The model formula is: 1.601 (if beyond up-to-7 criteria) - 0.428 (if albumin >35 mg/dL) - 0.464 (if presence of ORR) - 1.285.
ROC curve presented an AUROC 0.701 (p = 0.006) for the prediction of liver decompensation at 6 months. Using the cut-off value of -1.49, the model presented a sensitivity of 81% and a specificity of 72% for liver decom-pensation at 6 months.
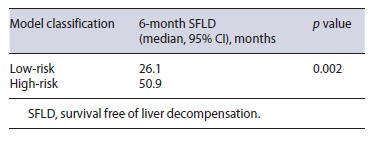
We used the cut-off value of -1.49 to classify patients as low-risk (<-1.49) and high-risk (≥-1.49) in accorance with the model developed. Patients classified ashigh-risk (n = 52) presented a higher prevalence of liver decompensation (35%) when compared with patients classified as low-risk (10%). This difference was statistically significant in chi-square test (p = 0.022). Furthermore, in Kaplan-Meyer analysis, high-risk patients pre-sented smaller overall survival free of liver decompensation when compared to low-risk patients (median, 26.1 vs. 50.9 months, p = 0.002) (Fig. 2; Table 4).
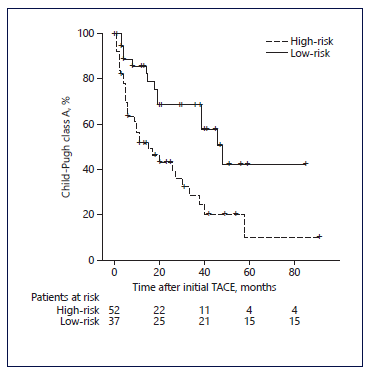
Fig. 2 Kaplan-Meier curves for the time to Child-Pugh class deterioration in patients with intermediate-stage hepatocellular carcinoma who underwent transarterial chemoembolization (TACE) as first-line treatment. Comparing patients classified as high-risk and low-risk in accordance with our model using the cut-off value of -1.49.
Transarterial Chemoembolization Treatment Outcome
The median TACE procedures per patient was 2 (IQR 1-3) and 41 patients (41%) presented ORR after first TACE. Fifty (51%) patients developed TACE refractoriness during follow-up and the median time to TACE refractoriness was 33 (IQR 11.7-54.3) months. Twelve (12.1%) patients presented post chemoembolization syndrome. No other major (CTCAE grade ≥3) complications related to TACE were registered.
In univariable analysis, up-to-7 criteria, number of nodules, total tumor size, and absence of ORR were identified as predictive factors associated with survival free of TACE refractoriness (Table 2). In multivariable analysis, beyond up-to-7 criteria and absence of ORR were identified as predictive factors of survival free of TACE refractoriness (Table 3).
Overall Survival
Presence of liver decompensation at 6 months after initial TACE was negatively associated with overall survival. Median survival was 38.7 (IQR 31.2-46.1) months for patients with Child-Pugh A and 12 (IQR 8.6-15.3) months for patients with Child-Pugh B/C at 6-month evaluation post first TACE procedure. This difference was statistically significant (p < 0.001). Moreover, albu-min <35 and beyond up-to-7 criteria were also negatively associated with overall survival in univariable analysis (Table 2). In multivariable analysis, beyond up-to-7 criteria (HR 2.4, p = 0.011) and albumin <35 mg/dL (HR 1.7, p < 0.042) were negatively associated with overall surviv-al (Table 3).
Discussion
Based on our findings, we suggest that baseline albumin <35 g/L, beyond up-to-7 criteria, and absence of response to initial TACE are predictors of liver decompensation to Child-Pugh B/C before TACE refractoriness, in patients with intermediate-stage HCC submitted to TACE. Moreover, early (6 months) liver decompensation was associated with baseline albumin <35 g/L, beyond up-to-7 criteria, and absence of response to initial TACE. Indeed, our model created using those variables was able to predict liver decompensation after initial TACE, and patients classified as high risk presented shorter survival free of liver decompensation.
Previous studies associated different markers of liver function and tumor burden as predictors of liver decompensation. However, those studies did not attempt to focus on predictors of liver decompensation before TACE refractoriness [18-20]. Furthermore, previous studies fail to analyze a specific population of patients with intermediate-stage HCC or a population exclusively treated with DEM-TACE. Identification of predictors of liver decompensation previous to TACE refractoriness is specifically useful in patients with intermediate-stage HCC, since the concept of therapeutic stage migration could be anticipated in such patients before TACE refractoriness. Furthermore, DEM-TACE, as opposed to conventional TACE or transarterial embolization is currently the most commonly used locoregional procedure in intermediate-stage HCC, with a better security profile and, in some studies, better efficacy [21-23]. Facciorusso et al. report a meta-analysis that evaluated the efficacy and safety of TACE versus bland embolization [23]. In this study no differences in survival were identified. However, despite the apparently worst security profile of conventional TACE versus bland embolization, DEM-TACE in particular appears to present lower rates of adverse events. Indeed, in our population, only twelve patients presented postembolization syndrome and no other complications were registered. Also, being a single-center study, TACE procedures were standardized and the choice of anticancer agents was uniformized.
The ORR is proposed as a prognostic tool and is useful in the selection of retreatment strategies [1]. Assessment for retreatment with TACE (ART) score is a scoring system using radiological response to initial TACE as a tool useful in the selection of patients that do not benefit from additional TACE [24, 25]. Our findings suggest ORR might also be useful to predict early liver dysfunction before TACE refractoriness.
Cumulative TACE procedure has been previously identified as a risk factor for liver decompensation [13, 26]. However, the number of TACE procedures and cumulative doxorubicin dose did not present an association with overall survival free of liver decompensation in our population. This could be explained by the type of locoregional therapy used. In our population, only DEM-TACE was used, contrary to previous studies. DEM-TACE presents less toxicity to liver function as opposed to conventional TACE [21, 22].
The concept of TACE refractoriness is useful to consider the opportunity to switch from TACE retreatment to ST [8, 17]. Previous studies demonstrated an association between liver decompensation and TACE refractoriness [18]. Indeed, in our population, beyond up-to-7 criteria and absence of ORR were independently associated with both liver decompensation and TACE refractoriness. As such, patients beyond up-to-7 criteria, with ab-sence of ORR, or with albumin <35 g/L not only have a smaller window of opportunity to initiate ST but have a greater probability of TACE refractoriness.
Several randomized controlled trials evaluating efficacy and safety of ST plus TACE or TACE alone in patients with intermediate-stage HCC have been done and others are currently in progress [11, 27]. Such studies have, so far, suggested a benefit in progression-free survival with this combination. However, the sub-population of patients who benefit the most from this combination is not yet described. We hypothesize that patients classified as high-risk of liver decompensation, using our model, might benefit from inclusion in such studies.
Furthermore, overall survival in patients with HCC treated with locoregional therapy is influenced by both recurrence and successive treatments. Previous studies demonstrate that a significant portion of patients experience HCC recurrence after locoregional therapy [28]. In patients with recurrence with advance-stage HCC, ST is the only palliate treatment available. Such patients are de-prived of this option in the presence of liver decompensa-tion. Patients classified as high-risk of liver decompensa-tion, using our model, present an increased risk of liver decompensation and in consequence, might have an in-creased risk of being deprived of adequate treatment in case of HCC recurrence.
As a retrospective study, there are some limitations from the presence of possible bias. Moreover, sample size limited the number of variables included in multivariable analysis, and only significant variables in univariable analysis were included. Further prospective large studies are necessary to confirm our results.
In conclusion, our findings suggest that in patients with intermediate-stage HCC and Child-Pugh class A, beyond up-to-7 criteria, albumin <35 mg/dL, and absence of ORR to initial TACE are predictors of early liver decompensation before TACE refractoriness. We created a model using those variables that is able to predict liver decompensation. Patients classified as high risk using this model have a smaller window of opportunity for alternative therapies and might benefit from inclusion in TACE plus ST combination therapy or ST monotherapy clinical trials.