Introduction
Laparoscopic and endoscopic cooperative surgery (LECS) is a procedure, which combines the advantages of endoscopy and laparoscopy. It was proposed by Hiki et al. [1] in 2008 as a technique to resect gastric subepithelial lesions (SELs). Before LECS was developed, SELs were generally treated by laparoscopic wedge resection (LWR). However, gastric SELs may not be recognized from outside of the stomach wall, making it difficult to accurately determine resection lines through LWR [2-4]. This can lead to incomplete or excessive resection, which may lead to increased recurrence or postoperative alterations of the stomach with gastric stasis [2, 4].
The first proposed technique was classical LECS that consists of endoscopic confirmation of the incision lines, followed by an endoscopic mucosal incision, while the seromuscular layer is incised laparoscopically. At the end, the incision line is sutured laparoscopically and the specimen is removed transabdominally [5, 6]. Classical LECS was proposed for SELs without ulceration, regardless of location. Its main benefits are allowing complete resection with minimal margins, preserving gastric motility and postoperative quality of life. Specially in esophagogastric junction SELs, LECS could avoid total or proximal gastrectomy [4]. However, classical LECS also has some limitations: possible peritoneal contamination with tumor cells or gastric juice (due to opening of the gastric wall) and requirement of advanced endoscopic and laparoscopic skills [7, 8].
To overcome these disadvantages and expand LECS for treatment of SELs with ulceration and gastric epithelial neoplasms, some modifications were developed, such as inverted LECS, combination of laparoscopic and endo-scopic approaches to neoplasia with nonexposure technique (CLEAN-NET), nonexposed endoscopic wall-in-version surgery (NEWS), and closed-LECS [4, 6, 7, 9].
Inverted LECS decreases the risk of intraperitoneal seeding by inverting the mass into the gastric lumen. Although this risk is not null, there is also intentional perforation. In addition to classical LECS, it allows the resec-tion of masses with less than 5 cm regardless of the location [6, 7, 10].
CLEAN-NET, NEWS, and closed-LECS are only suitable for SELs lesser than 3 cm. CLEAN-NET includes eversion of the mass and nonexposed full-thickness resection after seromuscular incision, preserving the continuity of the mucosa, that works as a barrier. The risk of mucosal laceration in lesions superior to 3 cm justifies the size limitation [9, 11]. NEWS stands on a “suture first and then cut” rule, including a full-thickness resection technique without intentional perforation [8, 12, 13]. The previous 2 types of modified LECS are not indicated for lesions located at the EGJ or pyloric ring. On closed-LECS and NEWS, lesions are retrieved by the transoral route [3, 4, 7, 9, 14, 15].
Endoscopic submucosal dissection (ESD) is the first-line treatment for gastric epithelial lesions without deep submucosal invasion, allowing en bloc resection, independently of size. Despite this, in SELs the risk of perforation is higher since the resection plan is deeper and is associated with long operation times and long learning curves [6, 7, 16]. Modified LECS procedures can also have a role in SELs located in the deep submucosa/muscle layer and in some early gastric cancers that would be technically difficult to treat with ESD. Specifically in the duodenum, where ESD is associated with a high perforation risk (20-30%), LECS is an attractive option since it might be safer than ESD and conventional surgery [4-6].
According to previous studies, LECS is a safe and feasible procedure, with a complication rate lesser than 5%, but there are no studies demonstrating if LECS is in fact better than ESD or conventional surgery, neither showing which is the better LECS procedure, among the classical and the modified ones [2, 4, 6, 17-19].
The aim of this systematic review and meta-analysis was to evaluate efficacy and safety outcomes of LECS for gastric, EGJ, and duodenal lesions and to compare LECS with competing techniques (ESD and conventional sur-gery) whenever possible.
Methods
This systematic review and meta-analysis followed the principles set in the Preferred Reporting. Items for Systematic Reviews and Meta-Analyses: the PRISMA Statement [20].
Study Search and Selection
Studies were identified through scanning of 3 electronic data-bases (MEDLINE through PubMed, Scopus, and ISI Web of Knowledge), with the last search performed on March 8, 2021. The search query for PubMed was (gastric OR stomach OR duodenal OR duodenum OR “esophagogastric junction”) AND (“laparoscopic and endoscopic cooperative surgery” OR “Laparoscopic-endoscopic cooperative surgery” OR LECS OR D-LECS OR “non-exposed endoscopic wall-inversion surgery” OR “non-exposed endoscopic wall-inversion surgery” OR “Nonexposed wall-inversion surgery” OR “non-exposed wall-inversion surgery” OR CLEAN-NET OR “combination of laparoscopic and endoscopic approaches for neoplasia with non-exposure technique” OR “Closed-LECS” OR “closed laparoscopic and endoscopic cooperative surgery” OR “non-exposure endoscopiclaparoscopic cooperative surgery” OR “inverted LECS”). Queries for other databases were adapted from this query. No time or language restrictions were made in this phase.
After removal of duplicates, two authors (J.T., S.B.) independently screened all titles and abstracts to exclude irrelevant studies. The full text of selected and relevant studies was then evaluated independently by the same two researchers according to the inclusion criteria described below. This phase was performed with Rayyan online platform [21]. Disagreements were solved by con-sensus between the authors or with the intervention of a third reviewer (D.L.) when required.
Inclusion criteria were: (1) retrospective or prospective, case-control, or cohort studies and clinical trials; (2) including patients submitted to laparoscopic and endoscopic cooperative surgery due to esophagogastric, gastric and/or duodenal lesions (single-arm studies as well as comparative studies with competing techniques were included); (3) evaluating at least one of the following efficacy or safety outcomes: R0/complete resection; need for conversion; procedure time; hospitalization time; recurrence; adverse events; and mortality. Exclusion criteria were: (1) case reports, reviews, letters to editor, surveys, and animal studies; (2) language other than English/Portuguese/Spanish/Italian/French; (3) studies pub-lished only in abstract form; (4) studies including less than 10 patients; and (5) studies with patient overlap with other included studies (in this case, the most informative reference was used).
Data Extraction and Quality Evaluation
Data extraction was performed by S.B. and D.L. Data extraction forms included (1) author, publication year, country, study period, study design, setting (2) population characteristics: numbers of participants, tumor location, histological subtypes; (3) type of resection techniques: LECS (NEWS, CLEAN-NET; closed-LECS), LWR, ESD, laparoscopic resection; and (4) outcomes: R0, need for conversion, procedure time (minutes), hospitalization time (days), recurrence, adverse events, and mortality. Data regarding costs and quality of life were also extracted whenever possible.
Quality evaluation was performed using the Newcastle-Ottawa Assessment Scale adapted, for cohort studies, by S.B. and D.L. [22]. Newcastle-Ottawa Scale ranges from 0 to 9 points in double-arm studies and from 0 to 6 points in single-arm studies. No specific value is assigned to high or low quality, although higher scores indicate higher quality and greater methodological aspects.
Data Synthesis and Statistical Analysis
Raw data regarding each outcome (number of events and total) were collected in order to calculate outcome prevalence and standard error. Effect measures included odds ratio (OR) for categorical variables and mean difference (MD) for continuous variables. For continuous outcomes, in some studies, median and range were transformed into mean and standard deviation through the methods proposed by the Cochrane collaboration and Hozo et al. [23, 24]. Meta-analysis was performed with Review Manager 5.4 software [25], using a random-effect model (when at least 3 studies were available for each outcome) [26]. Heterogeneity was evaluated with Cochran Q test and I2, being significant heterogeneity defined as p < 0.05 or I2 >40%, respectively. Subgroup analysis according to lesion was performed for 4 outcomes (need for conversion, procedure time, hospitalization time, and adverse events). Pooled mean for continuous variables and prevalence for categorical variables were calculated with OpenMetaAnalyst and Meta-XL, using a random-effect model [26, 27]. Publication bias was planned if ≥10 studies were included in comparative analysis for the primary outcomes (procedure time and adverse events).
Results
Study Selection
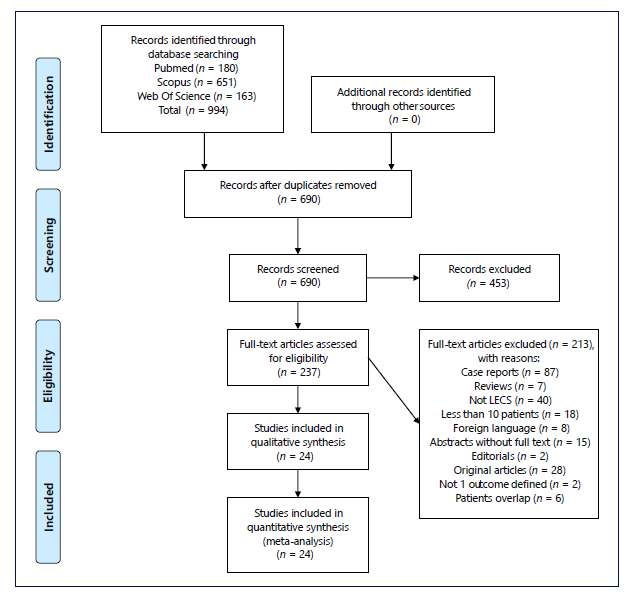
A total of 994 studies were identified through data base search. After removing the duplicates, 690 studies were screened regarding title and abstract and 453 were considered irrelevant. Therefore, the full text of 237 studies was assessed for eligibility by applying inclusion and exclusion criteria. Of those, 24 were included in this systematic review and all were included in meta-analysis. Study flowchart is shown in Figure 1, according to the PRISMA statement [20].
Study Characteristics and Quality Evaluation
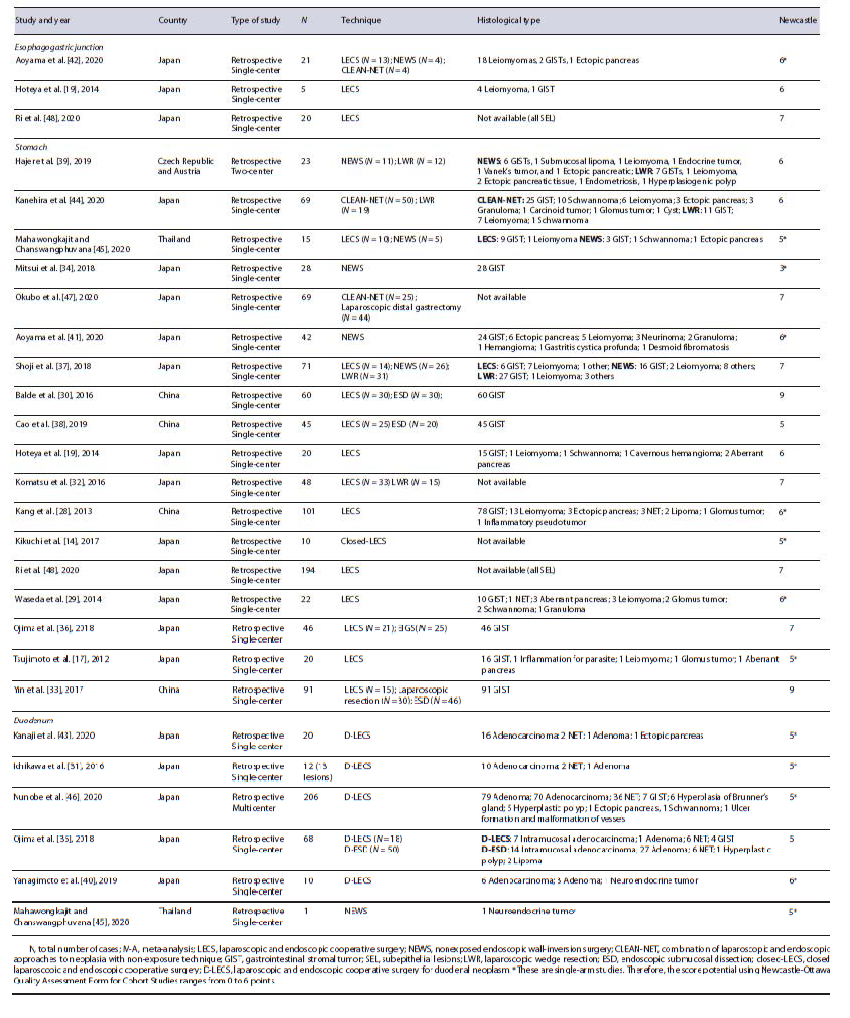
The main study characteristics are shown in Table 1 [14, 17, 19, 28-48]. Most of the studies (23) were from Asia (96%) and 22 studies (92%) were single-center. All studies were retrospective. The median Newcastle-Otta-wa Scale score of included studies was 6 (7 in double-arm studies and 5 in single-arm studies). A total of 1,337 lesions, from 1,336 patients, were included in the analysis, of which 46 (3.4%) were located in the EGJ, 974 (72.9%) in the stomach, and 317 (23.7%) in the duodenum. Half of the studies (12) were single-arm (of which 1 study was about lesions on the EGJ, 6 on the stomach, 4 on the duo-denum, and 1 had lesions on both the stomach and the duodenum [45]). The other 12 studies were comparative. One study reported data regarding costs [37] and another one regarding quality of life [47].
Comparative Studies
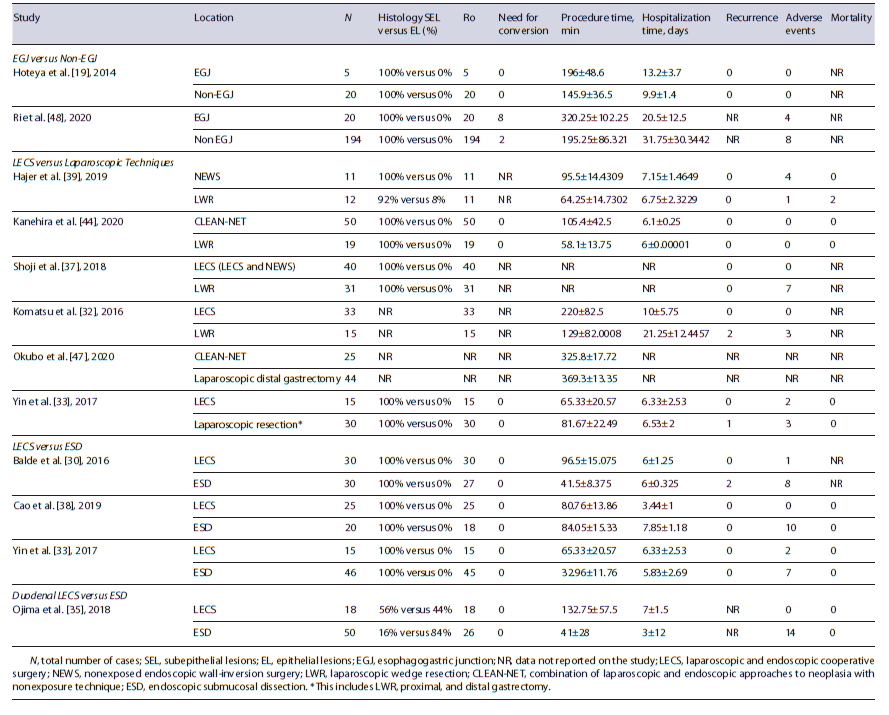
LECS versus Laparoscopic Techniques Six studies compared the outcomes of LECS and laparoscopic techniques (4 LECS vs. LWR [32, 37, 39, 44]; 1 LECS vs. laparoscopic gastrectomy [47]; 1 LECS vs. LWR and gastrectomy [33]) in gastric lesions. In meta-analysis, procedure time, hospitalization time, and adverse events were not significantly different between the two groups (shown in Fig. 2a-c), although there was a trend to longer procedure time in LECS’ group (MD 18.3 min, 95% CI: 23.1 to 60.3, I2 = 98%), shorter hospitalization time in LECS’ group (MD −0.37, 95% CI: −1.8 to 1.0, I2 = 74%), and lower adverse events rate in LECS’ group (4.0% vs. 13.1%, OR: 0.44, 95% CI: 0.04-4.65, I2 = 72%). Despite the tendency to longer procedure time in LECS’ group, in the 2 studies that compared LECS with Laparoscopic gastrectomy [33, 47], procedure time was higher in laparoscopic techniques’ group (shown in Table 2). R0 was 100% in LECS group and 99.6% (1/256) in LWR/laparoscopy’s group. R0 was not achieved in 1 case using LWR [39]. The conversion rate was 0% in both groups. Recurrence occurred in 3 of 107 cases (2.8%) in laparoscopic techniques’ group [32, 33] and in 0 of 149 cases (0%) in LECS’ group. Two deaths were reported among the 61 cases of the laparoscopic group [39], and 0 among the 76 cases of the LECS’ group (shown in Table 2). Both reported deaths were not related to the oncological disease neither its treatment. Meta-analysis was not possible in these last outcomes.
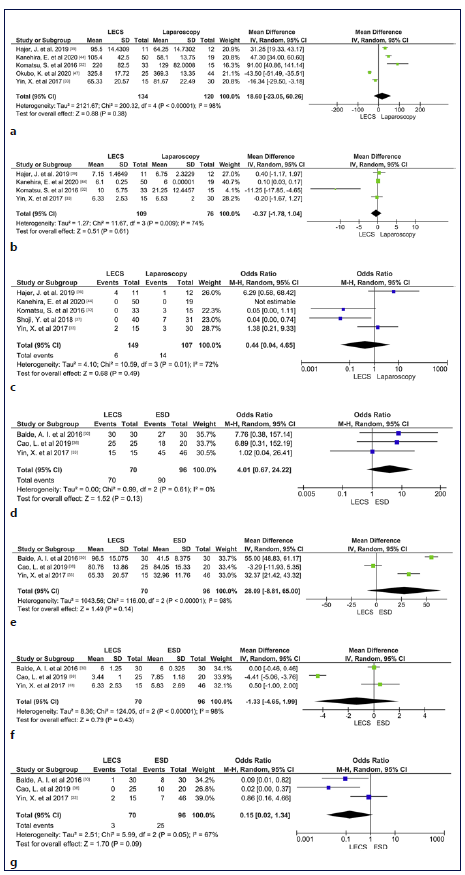
Fig. 2 a Forest plot of procedure time according to surgical technique (Laparoscopic Techniques vs. LECS). b Forest plot of hospitalization time according to surgical technique (Laparoscopic Techniques vs. LECS). c Forest plot of adverse event according to the surgical technique (Laparoscopic Techniques vs. LECS). d Forest plot of R0 according to the surgical technique (ESD vs. LECS). e Forest plot of procedure time according to the surgical technique (ESD vs. LECS). f Forest plot of hospitalization time according to the surgical technique (ESD vs. LECS). g Forest plot of adverse event according to the surgical technique (ESD vs. LECS). LECS, laparoscopic and endoscopic cooperative surgery; ESD, endoscopic sub-mucosal dissection; SD, standard deviation.
LECS versus ESD
Three studies compared the outcomes of LECS and ESD in gastric lesions [30, 33, 38]. In meta-analysis, R0, procedure time, hospitalization time, and adverse events were not significantly different between the two groups (shown in Fig. 2d-g), although there was a trend to higher complete resection rate in LECS’s group (100% vs. 94%, OR: 4.01, 95% CI: 0.67-24.22, I2 = 0%), longer procedure time in LECS’ group (MD 28.1, 95% CI: −8.81 to 65.00, I2 = 98%), shorter hospitalization time in LECS’ group (MD 1.33, 95% CI: −4.65 to 1.99, I2 = 98%), and lower adverse events rate in LECS’ group (4,3% vs. 26%, OR: 0.15, 95%CI: 0.02-1.34, I2 = 67%). The conversion rate was 0% in both groups. Recurrence was noticed in 2 of the 96 cases (2.08%) using ESD [30] and in 0 of the 70 cases using LECS. No deaths were reported in 106 cases (shown in Table 2).
Duodenal LECS versus ESD
One study compared the outcomes of LECS and ESD in duodenal lesions [35]. R0 was 100% in LECS’ group, but only 52% in ESD group, corresponding to 26 among the 50 cases. There was no need for conversion in any studies. Procedure duration and hospitalization time were higher in LECS’ group. Recurrence was not reported. ESD’s group accounted for a total of 14 adverse events (28%), whereas LECS had 0 adverse events among its 18 cases (0%). No deaths were reported (shown in Table 2). Meta-analysis was not performed due to a low number of studies.
Single-Arm Studies regarding LECS
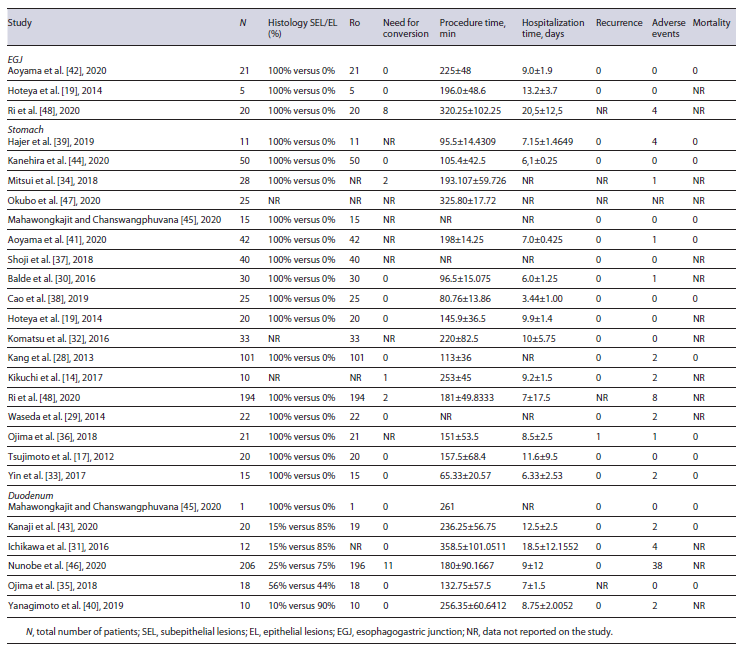
Half of all included studies were noncomparative LECS studies. Twenty-four studies provided data for the calculation of pooled efficacy and safety outcomes. This is shown in Table 3.
EGJ
Three studies provided data on 46 EGJ lesions [19, 42, 48]. R0 resection rate was 100% and pooled conversion rate was 11.3% (95% CI: 0-47.1%, I2 = 87%). The 8 conversion cases occurred in the same study [48]. Pooled mean procedural time was 246 min (95% CI: 185-307, I2 = 89%), and pooled mean hospitalization time was 13.7 days (95% CI: 8.0-19.3, I2 = 91%). Two studies reported recurrence rates [19, 42] and it was 0% (0 in 26 cases). Pooled adverse events’ rate was 7.1% (95% CI: 0-24.7%, I2 = 67%), with all 4 adverse events occurring in the same study [48]. Mortality was 0% (0 in 21 cases).
Gastric
Eighteen studies provided data on 702 gastric lesions [14, 17, 19, 28-30, 32-34, 36-39, 41, 44, 45, 47, 48]. R0 resection rate was 100%. Pooled conversion rate was 1.0% (95% CI: 0.4-2.2%, I2 = 0%). The 5 conversion cases occurred in 3 different studies [14, 34, 48]. Pooled mean procedural time was 158 min (95% CI: 118-199, I2 = 100%), and pooled mean hospitalization time was 7.3 days (95% CI: 6.6-8.0, I2 = 98%). Fifteen studies reported recurrence rates and it was 0.22% (1 in 455 cases). This case was reported by Ojima et al. [36], in an 85-year-old patient with a 90-mm lesion. Pooled adverse events’ rate was 3.5% (95% CI: 1.8-6.0; I2 = 44%), with a total of 24 adverse events reported in 677 cases. Mortality was re-ported only in 9 studies and it was 0% (0 in 300 cases).
Duodenal
Six studies provided data on 267 duodenal lesions [31, 35, 40, 43, 45, 46]. R0 resection rate was 95.7%. Incomplete resection was reported in 11 of 255 cases (4.3%), which included 2 studies: Kanaji et al. [43] and Nunobe et al. [46] . Pooled conversion rate was 4.3% (95% CI: 2.2-7.1; I2 = 0%). Need for conversion was reported in 11 cases, in Nunobe et al. [46] . Pooled mean procedural time was 229 min (95% CI: 176-281, I2 = 94%), and pooled mean hospitalization time was 10.1 days (95% CI: 7.5-12.7, I2 = 95%). Recurrence rate was 0% (0 in 249 cases). Pooled adverse events’ rate was 14.8% (95% CI: 5.8-25.6, I2 = 59%), with a total of 46 adverse events reported in 267 cases. Mortality was reported only in 3 studies [35, 43, 45] and it was 0% (0 in 39 cases).
Overall, pooled conversion rate was 2.5% (95% CI: 0.9-4.8%, I2 = 56%). Although there were no statistically significant differences in the conversion rate according to the location, there was a trend to higher conversion in EGJ lesions (11.3%, 95% CI: 0-47.1%, I2 = 87%), followed by duodenal lesions (4.3%, 95% CI: 2.2-7.1%, I2 = 0%) and gastric lesions (1.0%, 95% CI: 0.4-2.2%, I2 = 0%). This is shown in Figure 3. Pooled adverse events’ rate was 5.9% (95% CI: 3.1-9.5, I2 = 73%). Although there were no statistically significant differences in adverse events’ rate according to the location, there was a trend to higher rate of adverse events in duodenal lesions (14.8%, 95% CI: 5.8-25.6, I2 = 59%), followed by EGJ lesions (7.1%, 95% CI: 0-24.7, I2 = 67%) and gastric lesions (3.5%, 95% CI: 1.8-6, I2 = 67%). This is shown in Figure 3b. Pooled mean procedural time was 185 min (95% CI: 153-217, I2 = 100%). Mean procedural time was also not significantly different according to the location. However, the same trend was verified: higher procedural time in EGJ lesions (246 min, 95% CI: 184-307, I2 = 100%), followed by duodenal le-sions (229 min, 95% CI: 176-281, I2 = 94%) and gastric lesions (158 min, 95% CI: 118-199, I2 = 100%). This is shown in Figure 3c. Pooled mean hospitalization time was 8.3 days (95% CI: 7.6-8.9, I2 = 98%). Hospitalization time was significantly shorter in gastric lesions versus EGJ lesions (mean 7.3 vs. 13.7 days, 95% CIs of 6.6-7.9 and 8.9-19.3, respectively). There was higher hospitalization time in EGJ lesions (13.7 days, 95% CI: 8-19.3, I2 = 91%), followed by duodenal lesions (10.1 days, 95% CI: 7.5-12.8, I2 = 95%) and gastric lesions (7.3 days, 95% CI: 6.6-8, I2 = 98%). This is shown in Figure 3d.
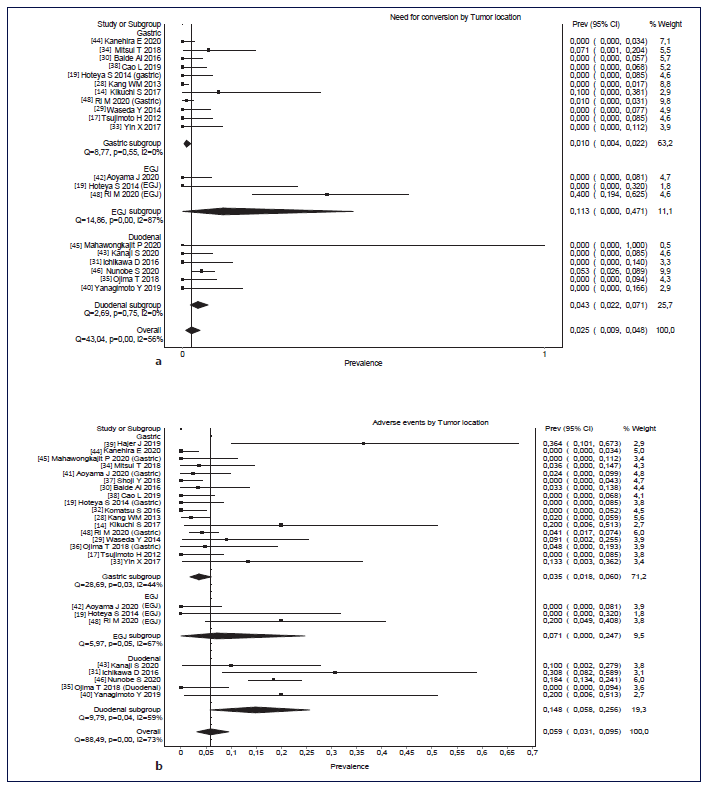
Fig. 3 a-d Rates of conversion and adverse events according to location (gastric, EGJ, and duodenal). Forest plots of mean hospitalization time and mean procedure time according to the location (gastric, EGJ and duodenal). a Rates of conversion according to the location (gastric, EGJ, and duodenal). b Rates of adverse events according to the location. c Forest plots of mean hospitalization time according to the location (gastric, EGJ, and duodenal). d Forest plots of mean procedure time according to the location (gastric, EGJ, and duodenal). EGJ, esophagogastric junction.
Costs
Shoji et al. [37] analyzed the operative costs of 3 techniques: LWR, LECS, and NEWS. NEWS was associated with a significantly lower mean total cost, followed by LECS, and being LWR the most expensive technique. The major cause pointed by the authors for these differences was the cost of suturing devices, such as laparoscopic linear staplers, which were less used in the NEWS’ group (p < 0.001). Authors also referred that, although a hand-sewn technique used on LECS and NEWS was cheaper, it increased the operative time, resulting in higher personnel and anesthetic expenses.
Quality of Life
Okubo et al. [47] evaluated postoperative quality of life after local resection (CLEAN-NET) and distal gastrectomy, using the Postgastrectomy Syndrome Assessment Scale (PGSAS-45) questionnaire and endoscopic evaluation at 1, 6, and 12 months after surgery. Authors reported significantly endoscopic differences 12 months after gastrectomy, with less esophageal reflux and residual gastritis in the group submitted to CLEAN-NET. CLEAN-NET subgroup also presented better clinical symptoms 12 months after procedure, reporting less indigestion, less dissatisfaction during meals, less dissatisfaction for daily life, and more amount of food ingested per meal, resulting in better nutritional status and body weight ratio.
Histology
Three studies reported histological evaluation of the surgical specimens in the EGJ [19, 42, 48]. All lesions were subepithelial (total of 46 lesions). Regarding gastric lesions, 15 studies reported histological evaluation of 847 surgical specimens [17, 19, 28-30, 33, 34, 36-39, 41, 44, 45, 48]. One lesion (0.1%) was epithelial [39] and the other 846 lesions (99.9%) were subepithelial. Six studies reported histological evaluation of retrieved lesions in the duodenum, making a total of 318 lesions [31, 35, 40, 43, 45, 46]. Seventy-six lesions (24%) were subepithelial and 242 (76%) were epithelial lesions. The location with higher number of epithelial retrieved lesions was the duodenum. This is shown in Tables 2 and 3.
Discussion
To the best of our knowledge, this is the first systematic review and meta-analysis comparing LECS with laparoscopic techniques (LWR and laparoscopic gastrectomy) and endoscopic techniques (ESD). We found that LECS is an effective and safe therapy for upper GI SELs, with high rates of R0 and low adverse events rates, shorter hospitalization time, and longer procedure time. Additionally, we found that, to date, there is no clear evidence of the benefit of LECS over ESD or LWR/laparoscopy namely in procedural/hospitalization time nor in R0/adverse events, in gastric lesions.
Regarding gastric lesions, no significant differences were found between LECS and preexisting techniques (ESD or LWR/laparoscopy) regarding any outcomes.
This might be explained by the high heterogeneity and low number of comparative studies. However, we could observe some trends in our results: LECS was associated with higher R0, shorter hospitalization time, and longer procedure duration, comparing to ESD. Comparing to laparoscopic techniques, hospitalization time was lower and procedure duration was higher when LECS was the chosen procedure. Although analyzing separately LWR and laparoscopic gastrectomy’s procedure duration, we noticed that LECS was shorter than those procedures using laparoscopic gastrectomies and longer than those using LWR. LECS was associated with fewer adverse events comparing with both laparoscopic and endoscopic procedures. Hajer et al. [39] stood out from the other 4 studies [32, 33, 37, 44], which compared adverse events between LECS and LWR/laparoscopy, having a marked deviation to the direction that favors LWR/laparoscopy. It is important to mention that it is a two-center study in which the two compared subgroups were from different hospitals and from different countries. Given that, we cannot assure that both groups have the same characteristics [39].
Regarding duodenal lesions, ESD was associated with nonsignificantly higher rates of incomplete local resection and adverse events, while LECS was associated with nonsignificantly longer procedure duration and hospital stay.
Hospitalization time was significantly shorter in gastric lesions compared to EGJ lesions. There were no statistically significant differences in conversion rate, mean procedural time, or adverse events’ rate according to the location. However, there was a trend to higher conversion rate and longer procedure duration in EGJ lesions and higher rate of adverse events in duodenal lesions.
The conversion rate tended to be higher in the EGJ subgroup because Ri et al. [48] reported 8 cases who needed conversion due to esophageal invasion and large defects after lesion resection (more than half of the circumference of the EGJ). This might explain the higher need for conversion presented by this study.
Two studies that reported the need for conversion in gastric lesions [14, 34] used 2 modified procedures: NEWS and closed-LECS, which include transoral removal of the lesion. Several lesions with more than 30 mm were reported, which require conversion to achieve adequate specimen retrieval. Therefore, this may partially explain the higher rates of conversion in these studies.
R0 was nonsignificantly lower in duodenal procedures (95.7% vs. 100%). This can be explained by several technical difficulties such as maintaining an adequate vision field, accessing the narrow duodenal lumen, holding the endoscope on position, and maneuvering it in such limited space [31, 43, 49, 50]. These difficulties can also explain the higher adverse events’ rates in the duodenum, comparing with other gastrointestinal locations.
Both procedure and hospitalization durations tended to be longer in the EGJ group, followed by the duodenum. This can be explained by the higher rate of conversion in these locations, as it will inevitably prolong the duration of the procedure and may lead to more complex techniques, such as proximal gastrectomies. EGJ lesions may be difficult to completely resect without excessive removal of surrounding tissues, increasing the conversion rate. Additionally, it also requires hand-suturing, which contributes to longer duration of the procedure [51].
Some other characteristics were approached. LWR was associated with higher operative costs than LECS and its modified procedures [37].CLEAN-NET was associated with better postoperative quality of life than distal gas-trectomy [47].
In another meta-analysis on this topic, Cai et al. [52] compared LECS with ESD and included Ojima et al. [36] in their meta-analysis. The procedure used by Ojima et al. [36] in the ESD arm (EIGS) did not fit in our definition of ESD, as it required the opening of the abdominal and gastric walls in order to deliver the endoscope and surgical instruments. Despite these differences, Cai et al. [52] reported higher incidence of complications and lower procedure time in ESD. Our results tended to the same conclusions.
This is the first systematic review and meta-analysis comparing LECS with laparoscopic and endoscopic techniques. We evaluated the safety and efficacy of LECS on three different locations: EGJ, stomach, and duodenum.
This systematic review and meta-analysis has some limitations. First, all studies were observational and retrospective reports (mainly single-center), as no randomized controlled trials exist on this subject, making them prone to selection bias. Although median quality of existing studies is good (6), the risk of bias is not null. Second, high heterogeneity was found in some outcomes, probably due to large variations among techniques. Despite being inspired by the same technique, modified LECS procedures have some differences. Moreover, our sample size was relatively small: we analyzed 1,337 lesions in this systematic review and most studies had a small number of cases, which can decrease their precision. Lastly, the number of relevant studies comparing LECS on EGJ and other locations as well as comparing duodenal LECS with ESD was insufficient to perform meta-analysis.
Conclusion
LECS can be a valid, safe, and effective treatment option in patients with EGJ, gastric, and duodenal lesions. However, we consider that treatment choice must be individualized, taking in account the experience of the center and the clinical expertise of the medical team involved. Prospective studies are needed to confirm if LECS is superior to other established techniques.