Introduction
Nonalcoholic fatty liver disease (NAFLD) is the most prevalent cause of chronic liver disease in the Western world, coupled with the worldwide increase in the prevalence of obesity and diabetes mellitus [1, 2]. Obesity not only associates with an increased risk for developing NAFLD [3, 4], it also associates with a more severe presentation, a higher risk for advanced liver disease, and liver cancer [3, 5].
It is estimated that the global prevalence of NAFLD has been increasing in the past decade and is currently around 24% of the general population worldwide [1].
One of the most relevant factors associated with the development of NAFLD is imbalanced dietary habits [1, 4, 6, 7]. In fact, NAFLD seems to associate with a high intake of calories, sodium, sugar, and fat, and a lower amount of nutritional dense foods present in their diet [6, 8, 9], as well as with a sedentary behavior [1, 8].
NAFLD guidelines recommend that the assessment of dietary and physical activity habits should be a part of the initial evaluation of NAFLD patients [10].
The Mediterranean diet is a dietary pattern strongly recommended for the management of NAFLD patients [4, 7, 10]. This dietary pattern is rich in fiber and polyunsaturated fatty acids. It is characterized by a high intake of fruit and vegetables, a moderate intake of low-fat dairy products and fish, and a low intake of red and processed meat [11]. This dietary pattern has demonstrated effectiveness in lowering body weight, improving clinical features of the metabolic syndrome and reducing the severity of liver disease [6, 12].
For the treatment of NAFLD a tailored approach combining diet and physical activity should be implemented [4, 7, 8, 10]. However, low adherence to lifestyle changes, specifically to dietary changes, is commonly reported amongst these patients [8]. Even though physician advice has demonstrated positive effects on weight loss, ideally the treatment of NAFLD patients should be managed by a multidisciplinary team, providing strategies to prevent relapses and weight gain [8, 13].
We aimed to assess the magnitude of weight loss in a group of NAFLD patients managed on a combined lifestyle intervention by a multidisciplinary team. We hypothesized that patients managed by the multidisciplinary team would achieve a higher weight loss compared to those managed solely by the hepatologist.
Materials/Subjects and Methods
Subjects
During 6 months, patients referred from primary care to the outpatient hepatology ambulatory clinic of a tertiary university hospital, with the diagnosis of NAFLD, were enrolled. The diagnosis of NAFLD was made on the presence of liver steatosis on ultrasound, after exclusion of significant alcohol intake (more than 20 g/day in women and 30 g/day in men), or of other causes of liver disease: chronic viral hepatitis B or C, primary biliary cholangitis, autoimmune hepatitis, primary sclerosing cholangitis, Wilson’s disease, hemochromatosis or α1-antitripsin deficiency. Patients were excluded if there was a history of treatment with potentially steatogenic drugs (such as steroids, high-dose estrogen, tamoxifen, methotrexate, or amiodarone) or if, prior to enrollment or during the 6 months of follow-up, patients had gastrointestinal bypass surgery or segmental small bowel resection. The severity of liver disease was evaluated by liver enzyme tests and transient hepatic elastography [10, 14]. Clinical data including liver biochemistry were collected.
The NAFLD Fibrosis Score (NFS) and Fibrosis 4 Score (FIB4) were calculated to evaluate fibrosis severity, and advanced fibrosis was considered when FIB4 >2.67 and NFS >0.675 [15, 16]. Advanced fibrosis was considered if liver stiffness measurement, measured by transient elastography (FibroScan®), was ≥11.1 kPa [17].
Lifestyle Counselling and Nutritional Assessment
All NAFLD patients were invited to be managed in a multidisciplinary group (hepatologist, dietitian and psychologist - MdT group). Clinical data were collected by two experienced hepatologists, who used a standardized protocol to minimize bias on data collection. At baseline, a dietician performed a complete nutritional assessment. General lifestyle recommendations were given and included written resources containing healthy eating guidelines and tips, basic information on portion size, exchange tips and general dietary recommendations. Other specifications of dietary advice included alcohol abstinence.
Baseline physical activity was assessed using the International Physical Activity Questionnaire [18]. Patients were also encouraged to increase their physical activity, with an incremental approach of exercise in everyday life, aiming at 150 m/week [10].
Patients who agreed to enroll in the multidisciplinary approach (MdT) also received a structured personalized dietary plan, with 5-7 meals a day, aiming at caloric restriction (-500 kcal) to promote weight loss. This structured nutritional plan was based on the Mediterranean diet and was individualized according to nutritional needs, the presence of other dietary restrictions alongside NAFLD, and the personal preferences and food habits of the patients. Patients who declined long-term management by the dietitian still received general lifestyle recommendations and were designated as conventional treatment group, maintaining management solely by the hepatologist. This group was used as a comparison with the MdT group.
Regular appointments at 1, 3, 6, and 12 months were provided by a dietitian. During appointments, adherence to recommendations was monitored [19]. Psychological support was offered during follow-up to increase the motivation to adopt lifestyle changes and to help coping with difficulties.
Anthropometric data were collected with participants wearing light clothes and barefoot; current weight was measured using a calibrated scale, and height was assessed using a stadiometer. History of weight loss in the previous 6 months was recorded. Waist circumference was measured halfway between the inferior rib and the iliac crest, and abdominal circumference was measured at the umbilicus level [20]. Body mass index (BMI) was defined as an individual’s weight in kilograms divided by the square of height in meters (kg/m2) and classified according to the World Health Organization. Body fat mass (%) was assessed using a single-frequency bioimpedance analyzer (Omron BF350).
During follow-up, patients who presented a clinical condition that implied a modification of dietary pattern and/or had a clinical condition that impacted directly on nutritional status (i.e., pregnancy, newly diagnosed cancer or gastrointestinal disease, hospital stay, bariatric surgery) were excluded. Patients who did not comply with scheduled appointments (MdT or conventional treatment group) were also excluded from the analysis.
Statistical Analysis
To determine a significant difference in both groups for α = 0.05 and a power of 0.8, we estimated a minimum sample of 21 participants in each group. Normal distribution was assessed using histograms, boxplots and the Shapiro-Wilk test. Continuous variables were summarized as mean and standard deviation (mean ± SD) or median and interquartile range (IQR) when the distribution was not normal. Categorical variables were summarized using frequency and percentage. For normal distributed variables, the χ2 or Fisher exact test for 2 × 2 tables was used for independence of categorical variables and the Student t test or ANOVA, with multiple comparisons adjusted with Bonferroni correction, was used for continuous variables. For each analysis, unadjusted and adjusted odds ratio (OR) and the corresponding 95% confidence interval (CI) and the p value were reported. The significance level was set at 5%. All data analyses were performed with IBM® SPSS® software, version 26.0.
Results
Population Characterization
From the 139 patients assessed, 50 were excluded for having other identifiable causes of liver disease. During follow-up, 8 patients were excluded due to the occurrence of pregnancy, scheduled bariatric surgery and diagnosis of cancer.
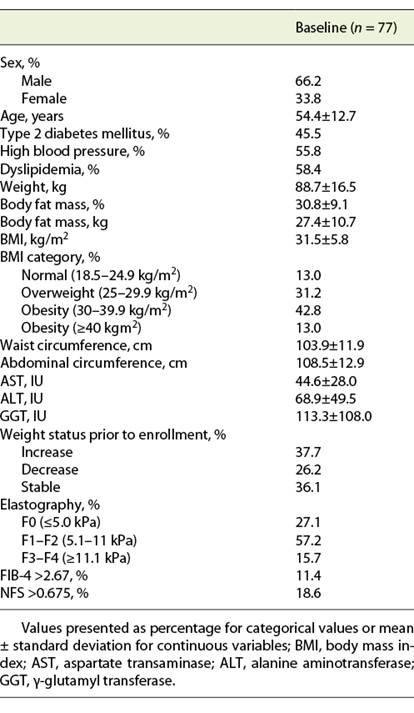
Seventy-seven patients with NAFLD were enrolled, of whom 51 were males (66.3%). At baseline, BMI was 31.5 ± 5.8 kg/m2, with 31.2% of patients being overweight and 55.8% obese. Advanced fibrosis was present in 15.7% of patients when assessed by elastography, and in 11.4 and 18.6% of patients, according to FIB4 and NFS, respectively (Table 1).
From the 77 patients, 14.3% (n = 11) declined to be managed by the MdT (conventional treatment group). There were no significant differences in baseline characteristics, namely age, sex, comorbidities, baseline BMI and severity of liver disease, between groups.
At baseline, none of the patients included in both groups presented moderate or vigorous physical activity levels.
Anthropometric Evolution
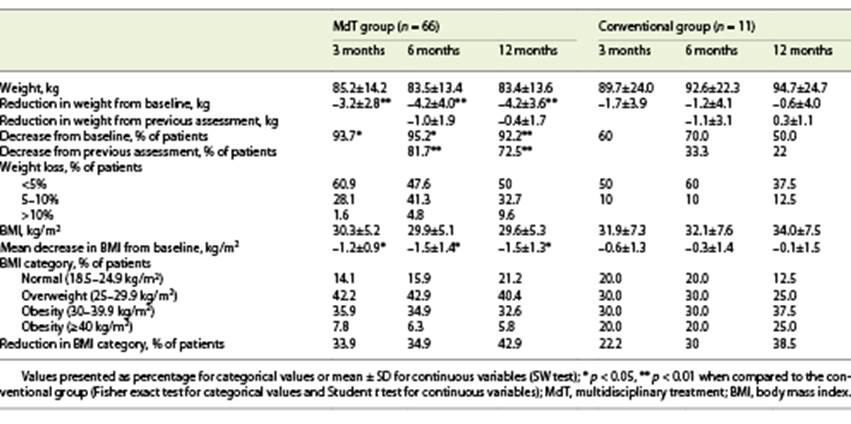
After 3 months, 89% of patients lost weight, with a mean weight loss of 3.1 kg. At 6 months, 75.4% maintained the weight lost, with a mean additional weight loss of 0.7 kg. At 12 months, 65% of patients decreased their weight, but only with a mean additional reduction of 0.3 kg. When comparing groups, we witness a higher degree of weight loss in the MdT group. Indeed, in the MdT group, at 3 months, 93.7% of patients lost weight at 3 months (vs. 60% in the conventional group; p = 0.001); 81.7% of patients continued to lose weight at 6 months (vs. 33.3% in the conventional group; p = 0.005) and 72.5% at 12 months (vs. 22% in the conventional group; p = 0.006). Also, in the MdT group, comparing with baseline, 95.2% of patients had a lower weight at 6 months (vs. 70% in the conventional group; p = 0.003), and 92.2% at 12 months (vs. 50% in the conventional group; p = 0.008).
At 12 months, 32.7% of patients in the MdT group had a weight reduction between 5 and 10%, as compared to only 12.5% of patients in the conventional treatment group (p = 0.007). None of the patients in the conventional treatment group presented a weight loss higher than 10%, compared with 9.6% of patients in the MdT group at 12 months (Table 2).
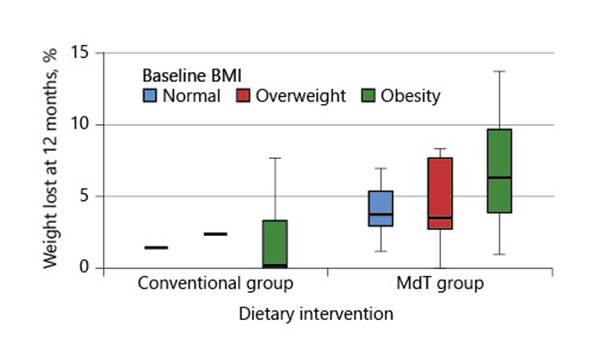
The presence of comorbidities (diabetes, high blood pressure, or dyslipidemia) was not associated with weight variation or percentage of weight loss. Also, age, sex, presence of comorbidities, and severity of liver disease were not associated with the magnitude of weight loss at 12 months. Furthermore, there was no association between previously reported weight loss or baseline BMI with the magnitude of weight loss achieved during the intervention period, although there was a tendency for patients with a higher baseline BMI to achieve higher weight loss (Fig. 1).
Evolution of Markers of Liver Disease Activity, and Evidence of Fibrosis Severity by Elastography
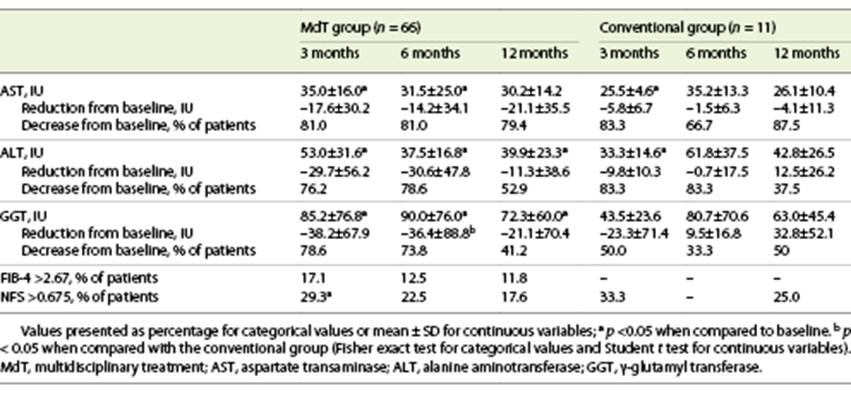
Liver enzymes decreased in most patients, with the effect being attenuated over time: decrease in AST in 81.3, 79.2, and 81% of patients, ALT in 77.1, 79.2, and 50%, and GGT in 75, 68.8, and 42.9%, at 3, 6, and 12 months, respectively.
The MdT and conventional treatment groups did not show any significant difference in baseline liver enzymes. However, although both groups experienced a reduction in liver enzymes, the mean values at 3, 6, and 12 months improved only in the MdT group, with significant reductions. The group of patients of the MdT group who presented weight loss >10% at 12 months presented a lower median ALT (20 IU) when compared with patients with a lower weight loss (29 IU 5-10%; 43 IU <5%, 41 IU in patients with no weight loss; p = 0.042). Globally, 52.2% of patients showed an improvement in liver fibrosis measured by FIB4 and 49.2% by NFS, without significant differences between MdT and conventional treatment groups (p > 0.05) (Table 3).
In a group of 24 patients who performed more than one transient elastography during follow-up, an improvement was verified in 70.8%, with a significant reduction of mean fibrosis values between baseline and 6 months (10.1 ± 6.5 vs. 8.5 ± 6.7 kPa; p = 0.048) in the MdT group. In the conventional treatment group, all patients maintained an F2-F3 classification and in the MdT group F0 patients increased from 23.8% at baseline to 38.1% with a reduction in F1-F2 from 52.4 to 42.9% and F3-F4 from 23.8 to 19% of patients after 6 months (p > 0.05).
Discussion
Our results suggest that a multidisciplinary approach is effective in inducing weight loss and in maintaining the weight loss during the 12-month follow-up.
In this study we evaluated the efficacy of a multidisciplinary team on the implementation of lifestyle changes, regarding the adoption of a more balanced dietary pattern and an increase in physical activity. The results of the present study suggest that a multidisciplinary approach is effective. However, regular follow-up by the multidisciplinary team is needed since patients who had only one nutritional appointment and maintained management solely by the hepatologist presented lower weight loss and regained almost all the lost weight.
Regarding an effect on liver fibrosis, in the subset of 24 patients that performed more than one transient elastography, we witnessed a trend to a decrease in mean kPa. We could not demonstrate an improvement of liver fibrosis with the non invasive scores FIB-4 and NFS. However, the time interval of 12 months is probably not enough to accomplish a significant reduction in fibrosis degree.
Our results are consistent with previous studies [21, 22] that have demonstrated that tailored nutritional intervention integrated in a multidisciplinary approach is effective in reducing body weight and improving liver enzyme tests and steatosis in NAFLD patients.
It has been demonstrated that fat or carbohydrate content of the dietary intervention has different influences on liver disease severity scores and assessment of steatosis [23]. However, instead of an intervention of specific nutrient, dietary patterns have emerged as a better option to study the impact of dietary intervention on the development or progression of liver disease [24].
Although some dietary strategies have demonstrated benefits in weight loss, such as the classic hypocaloric low-fat/low-carb diet [25, 26], and recently ketogenic diet [27], and intermittent fasting [28], the Mediterranean dietary pattern is the one recommended by the European Association for the Study of the Liver in NAFLD guidelines. Indeed, several epidemiological and interventional studies assessing the Mediterranean diet showed a beneficial effect on BMI reduction and long-term maintenance of body weight, as well as on triglycerides, cholesterol and glycemia, and liver enzymes [7, 29-32]. However, directives on how to implement it and the magnitude of caloric restriction required remains unclear. Also, additional changes to the traditional pattern, such as recommendations on alcohol intake, remain unclear [33].
In this study, we were able to demonstrate that with the modification of the traditional Mediterranean dietary pattern, the group of patients thus treated demonstrated a higher weight loss, a higher reduction in liver enzymes and a higher and more sustained weight loss in comparison with patients with general nutritional counseling.
One of the strengths of this study is the demonstration that a multidisciplinary approach is more effective than standard counseling alone. However, and although it has been demonstrated that educational interventions improve readiness to change dietary habits [34], we cannot exclude the degree of bias resulting from the fact that those in the conventional treatment group could be the less motivated for a change in their lifestyle. Additionally, our study has a relatively small sample size. Another limitation of this study is the inability to quantify the motivation for change in both groups, even though a psychological intervention was carried out.
Previous studies with liver biopsy were able to demonstrate that weight loss was very effective in improving all histological parameters of NAFLD, much more significantly than any pharmacological intervention [35]. In the present study, because performing a liver biopsy for the sole purpose of this research was considered unethical, histology was not available, what precludes us from evaluating in more detail the effect of the MdT.
This study also contributes to explain the reason why the placebo arms of the NAFLD/nonalcoholic steatohepatitis treatments frequently have a significant improvement in their liver disease. In fact, patients enrolled in critical trials often start to receive more attentive lifestyle counseling and gain motivation to change their lifestyle with consequent weight loss [36]. Education interventions improve anthropometric parameters, metabolic syndrome and cardiovascular disease, liver disease, and also health-related quality of life [13, 34].
In conclusion, this study demonstrates that a multidisciplinary approach is effective in promoting a Mediterranean dietary pattern, reducing body weight, and maintaining that weight loss.
Even though differences in baseline motivation cannot be excluded, patients of the conventional treatment group with one session of nutritional counseling were able to achieve weight loss. This study shows that one session might not be enough to guarantee the amount of weight loss necessary to improve liver steatosis/fibrosis. This reinforces the need to maintain/create adequate multidisciplinary groups to guarantee an adequate care and management of these patients, since often clinicians do not have either time or resources to support behavior change [37].
Further studies incorporating motivational and quality of life questionnaires coupled with dietary intervention are needed to better characterize NAFLD patients and to delineate more tailored lifestyle interventions.