Introduction
Duplication cysts can occur anywhere along the gastrointestinal tract, with the duodenum being the least common location and accounting for 2-12% of cases [1]. There are various theories regarding the etiology of duodenal duplication cysts (DDCs), all based on a defect during embryological development [2, 3]. Most patients with DDCs experience symptom onset in the first decade of life [1, 2]. The initial presentation is variable, but the most commonly reported clinical manifestations are abdominal pain and nausea/vomiting [1, 2]. Pancreatitis (acute or chronic), hepatitis, cholestasis, failure to thrive or weight loss, gastrointestinal bleeding, cyst infection, and intussusception can also occur [1, 2]. Few cases of malignant transformation have also been documented in the setting of gastric mucosa heterotopia within the DDC [4]. Diagnostic tools include imaging and endoscopy [5, 6]. Symptomatic patients traditionally undergo surgical treatment of the cyst, with few cases of endoscopic marsupialization reported in the literature [2, 6-15].
We present two cases of DDCs clinically manifesting in adults with acute pancreatitis that were diagnosed and treated endoscopically.
Case Reports
Case 1
A 23-year-old female patient was admitted to the emergency department complaining of acute intense epigastric pain. She mentioned that since the age of 17, she had had multiple similar episodes of self-limited abdominal pain. The patient had no previous medical history nor medication.
Standard diagnostic workup of acute pancreatitis was performed without findings of severity. Magnetic resonance cholangiopancreatography excluded choledocholithiasis and revealed a cystic lesion in the duodenum wall (Fig. 1).
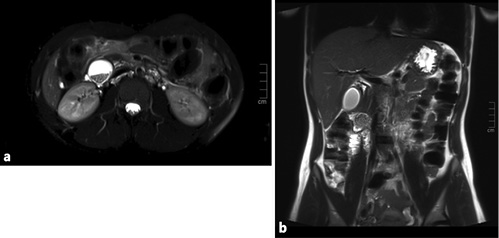
Fig. 1 Case 1. Magnetic resonance cholangiopancreatography images. a Axial T2FS section at the level of the periampullary duplication cyst, with stones in its lumen. b Coronal T2 section, with a gallbladder without lithiasis and the cyst at the duodenal wall.
Endoscopic ultrasound confirmed a 35-mm subepithelial lesion located in the second portion of the duodenum, whose proximal limit was immediately distal to the ampulla of Vater and adjacent to, but independent of, the uncinated process. It had a small quantity of fluid and calcifications. No perilesional lymph nodes were detected. Biliary and pancreatic ducts and pancreatic parenchyma were normal.
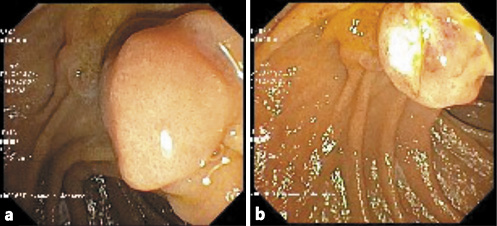
A duodenoscopy was performed using a duodenoscope. In the second portion of the duodenum, immediately distal to the major papilla, a bulging subepithelial lesion was detected (Fig. 2a). Using a diathermic snare (small oval stiff CaptivatorTM; Boston Scientific), deroofing of the cyst, away from the papillary region, was made (ERBE settings: ENDOCUT Q, effect 3, duration 1, cutting interval 6) and a large fragment of the lesion was resected (Fig. 2b). The cystic cavity was completely filled with debris that was removed with the snare and vigorous irrigation (Fig. 2c). No complications occurred.
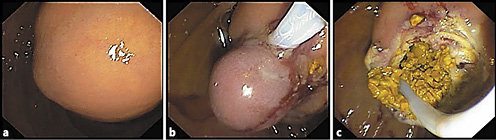
Fig. 2 Case 1. Endoscopic cyst deroofing. a Duodenal bulging subepithelial lesion. b A large fragment of the lesion was resected with a diathermic snare. c The cystic cavity was filled with stones, which were removed with the snare.
Histological assessment of the resected specimen was compatible with the diagnosis of DDC, with the surface covered by duodenal-type mucosa and with a single wall, without evidence of heterotopia, dysplasia, or neoplasia.
An endoscopic re-evaluation was performed 1 month later and no residual lesion was seen. No clinical or endoscopic recurrence was detected at 1-year follow-up.
Case 2
A 41-year-old female was admitted to the emergency department due to acute intense abdominal pain located in the epigastrium and right hypochondrium. No other associated symptoms were reported. One month earlier, the patient had been submitted to laparoscopic cholecystectomy due to acute pancreatitis, assumed as having a lithiasic etiology.
Computed tomography revealed a 20-mm cystic endoluminal image close to the major papilla, with dense content, suggesting lithiasis.
The patient was submitted to exploratory laparoscopy, and suture of Luschka canaliculi was performed. Nine days later, due to biliary drainage by the abdominal drain, she started a parenteral diet and was submitted to a second exploratory laparoscopy and new suture at the same level. Due to persistence of biliary drainage, 4 days later, the patient was referred for endoscopic retrograde cholangiopancreatography. In the second portion of the duodenum, immediately distal to the papillary orifice, a large bulging subepithelial periampullary lesion with a 20-mm major axis was detected (Fig. 3a). Biliary cannulation was performed and the cholangiogram showed a nondilated biliary tree without lithiasis and, at the level of the cystic stump, a contrast leak was confirmed (Fig. 3a). A biliary sphincterotomy was performed and a 7 cm × 8.5 Fr plastic stent was inserted (Fig. 3b, 4b), allowing the resolution of bile leak. The soft duodenal lesion was approached and easily fenestrated with a biopsy forceps. A cystogram was performed, ruling out communication with the biliopancreatic tree (Fig. 4b). Fenestration was expanded with a sphincterotome (ERBE settings: ENDOCUT I, effect 2, cutting duration 3, interval 3; Forced coag 50 W, effect 2), allowing the exit of mucinous fluid and debris (Fig. 3c). The procedure was uneventful.
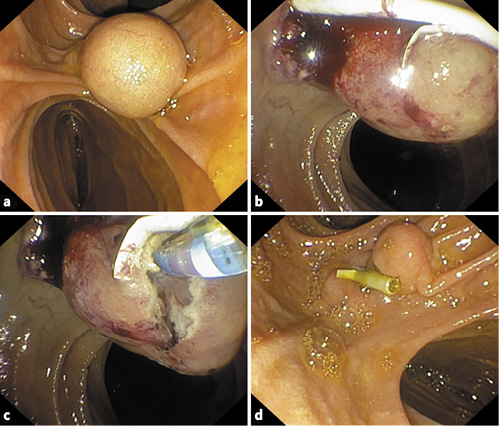
Fig. 3 Case 2. Endoscopy findings. a Duodenal bulging subepithelial lesion. b Status after biliary sphincterotomy and biliary plastic stenting. c Sphincterotome expanding the incision of the fenestration and allowing the exit of mucinous fluid. d One-month endoscopic re-evaluation showing the biliary stent in situ and a small residual lesion.
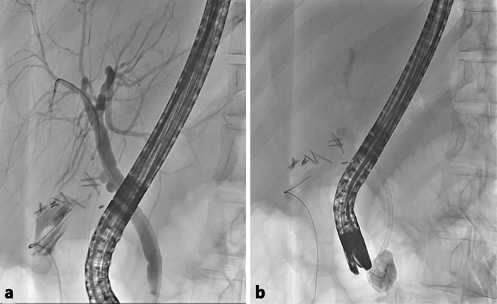
Fig. 4 Case 2. Endoscopic retrograde cholangiopancreatography findings. a Cholangiogram showing a cystic stump leak. b Biliary plastic stent placement and cystogram.
One month later, endoscopic retrograde cholangiopancreatography was repeated. A residual small duodenal fold was seen (Fig. 3d). The biliary stent was removed and a cholangiogram was repeated, showing no evidence of biliary leak.
A year and a half later, the patient remained asymptomatic, and upper endoscopy (Fig. 5) with a macrobiopsy showed no cyst recurrence.
Discussion
DDCs represent a minor part of all gastrointestinal tract duplications [5]. The pathogenic mechanism seems to be associated with duodenal epithelial pinching during the outgrowth of the dorsal pancreatic bud or epithelial sequestration [2, 3].
The clinical presentation of DDCs depends on the size and location of the cyst and its relationship with nearby anatomical structures. They are generally diagnosed during childhood, with obstructive symptoms being the main complaints [2]. In these two cases, as in others rarely reported before [7, 8, 16-18], the diagnosis was made with an acute pancreatitis episode occurring during adulthood. In case 1, we can assume that DDC was the cause of acute pancreatitis, as other etiologies had been excluded. In case 2, the etiology of the acute pancreatitis cannot be fully confirmed, but apart from the lithiasis, the DDC could have also contributed to its development. Several mechanisms related to the presence of a DDC may explain pancreatitis in these patients: (1) transient, mobility-related obstruction of the major papilla outflow by the cyst, (2) and/or compression of the pancreatic duct by a large cyst [2].
DDCs are usually large and located within the second portion of the duodenal wall. They closely mimic a choledochocele [6, 9], and imaging and endoscopic [5, 6] tools are important for differentiating these entities. Typically, peripapillary DDCs are located distal to the papilla with no communication with the biliopancreatic structures, while the choledochocele is part of the bile duct, bulging proximally to the papillary orifice [8]. In the cases presented herein, imaging was not sufficient for the diagnosis, probably because of DDCs’ rarity and variety in terms of clinical presentation and radiological findings. Endoscopically, the position of the papilla helped distinguish between both. In endoscopic ultrasound, DDCs are documented as being lined by duodenal mucosa and having a distinct muscle layer, whereas choledochoceles are lined by either bile duct or gallbladder mucosa and lack a muscle layer. The internal content may be anechoic or hypoechoic. As in these cases, DDCs can contain thick mucinous material, septations, fluid levels, and debris. In case 1, the presence of enteroliths compromised a complete ultrasonographic evaluation. Enteroliths inside DDCs have been previously reported and may be attributed to food stasis and alkalinity [19]. Pathological assessment, as performed in case 1, helps confirm the diagnosis and exclude (pre)malignant transformation.
The treatment of DDCs was traditionally surgical [3], which was associated with a high morbidity, due to the proximity of the cyst to the papilla and the biliopancreatic confluence. It might even end up with a pancreaticoduodenectomy. Since 1984, successful endoscopic therapy for intraluminal duplication cysts, using different methods for creating a wide and persistent opening of the anterior portion of the cyst, has been reported [6-15]. It can be done by resecting the cyst roof using a standard polypectomy snare or by a large marsupialization in the roof using a sphincterotome, a needle knife, or an endoscopic dissection knife. Endoscopic cyst management has been shown to have a high technical and clinical success, with faster recovery times compared to surgery [8, 20]. The only complication previously reported during endoscopic treatment of DDCs is bleeding, successfully managed during the procedure [8]. The theoretical disadvantage is the partial resection of the cyst, which does not allow to totally exclude the presence of gastric metaplasia or dysplasia. To overcome this limitation, as much as possible of the cyst wall should be excised during endoscopic treatment. For this reason, snare excision of the bottom should be the preferred technique, and a 1-year follow-up endoscopy is recommended.
These cases highlight the need to consider DDC in the differential diagnosis of acute pancreatitis in adults and the possibility of endoscopic cyst marsupialization as its first-line treatment.