Introduction
Pancreatic cancer is one of the most lethal malignant neoplasms. Despite all recent advances, pancreatic cancer is still diagnosed mostly at advanced stages, with 80-90% of the patients presenting unresectable disease at diagnosis, which contributes to a 1-year survival rate after diagnosis of 24%, and a 5-year survival rate of only 9% [1, 2].
As previously described, pancreatic cancer’s natural history mostly illustrates the behavior of its main histologic type - ductal adenocarcinoma, which comprises about 85% of all pancreatic cancers. However, pancreatic cancer is not always this dark since there are other histologic subtypes that can harbor a favorable prognosis.
Solid pseudopapillary neoplasm (SPN), first described by Frantz in 1959, also known as “Frantz’s tumor” [3], is an example. Described as a rare “low-grade malignant pancreatic tumour” by the 2019 WHO Classification of Tumours of the Digestive System [4], SPNs are unique neoplasms, and there is still insufficient information about them in the literature. The few articles that address this topic, do it retrospectively, by means of case series or case reports, making the clinical available knowledge on this issue limited. This article aims to summarize the most relevant topics about this entity.
Methods
A literature search on PubMed was conducted in January 2021, with backward citation conducted as well. Additionally, two articles were intentionally searched on Google, using, respectively, the queries “Solid Pseudopapillary Neoplasms of the Pancreas Hospital São João” (which represents this institution’s experience) and “World Health Organization Classification of Tumors of the Digestive System.”
The PubMed search strategy was sensitive to texts and abstracts, using the query: “((solid) AND (pseudopapillary) AND ((neoplasm) OR (tumor))) AND ((pancreas) OR (pancreatic)) AND ((management) OR (Clinical-pathological) OR (Clinicopathological) OR (features)).”
Eighty-four appealing titles and abstracts, considering the scope of the paper, were first selected from the PubMed search’s including 596 results. After full text reading, 40 were excluded due to repeated information or no clinical relevance. After searching the references from the 44 studies included, 10 more were added. Finally, the 2 studies from the google search and 2 studies suggested by peer review were also added, totaling 58 articles included in this review.
Tumorigenesis
SPN is a rare “low-grade malignant pancreatic tumour” (WHO classification 2019) [4] accounting for less than 10% of the cystic tumors of the pancreas, and up to 1-2% of all pancreatic tumors [5]. More than rare, it is a unique neoplasm due to its intriguing origin, since its histogenesis still remains obscure and hypothetical owing to the fact that its phenotype does not correlate to any specific lineage: neither pancreatic (either acinar or ductal), nor epithelial, neuroendocrine, or histiocytic. A few hypotheses have been formulated, but there is a recent and apparently more evidence-supported hypothesis stating that this neoplasm is derived from genital-ridge-related cells that were attached to the pancreatic tissue during development. It is supported by the finding that most extra-pancreatic SPNs are not associated with ectopic pancreatic tissue, suggesting a nonpancreatic origin, as well as by the fact that ovarian SPNs are morphologic and genetically similar to pancreatic SPNs [6-8].
From a molecular perspective, SPNs are intriguing as well. Pancreatic ductal adenocarcinoma common genetic alterations (including KRAS, TP53, CDKN2A and SMAD4) are not present in SPNs. Genetic alterations in SPNs are rather related to an aberrant Wnt signaling, which seems to be the hallmark of these neoplasms. In fact, virtually all SPNs are associated with hyperactivation of the Wnt signaling pathway and almost all due to acquired activating mutations of the CTNNB1 oncogene, which result in nuclear β-catenin accumulation and downregulation of E-cadherin. The latter leads to loss of cohesivity between tumor cells, giving rise to the degenerative changes underlying cystic alterations of SPNs. Other candidate genes implicated include TFE3 and LEF1 [9]. Nonetheless, these genetic alterations are not specific enough to be considered pathognomonic [6, 10-12].
One of the immunohistochemical hallmarks of this neoplasm is the expression of progesterone receptors (PRs). Considering its higher prevalence among young women and taking into account the larger tumor mean size denoted with higher progesterone serum levels, such as in pregnancy, it could be hypothesized that PRs play a role in tumoral cell replication [13].
Clinical Features
Because it is a rare disease, most publications are small case series and cannot accurately illustrate the epidemiological behavior of this neoplasm. There are, however, two studies reviewing all SPNs cases documented, on a specific timeline, in the English [14] and both English and Chinese [5] literature. We chose these two publications as models when describing clinicopathological characteristics. In summary, both studies [5, 14] agree that patients are predominantly young females (male/female ratio of 1:5.3-9.78), mostly aged between 20 and 30 years old.
A few studies investigated possible differences between SPN in males and females, and a few different clinicopathological features have been shown [15-17]. Most importantly, it seems that males are diagnosed at a later age (Wu et al. [16] reported a peak in incidence at approximately 64 years old) and that these have a poorer prognosis (Table 1).
SPNs can also occur at the pediatric age, but these are otherwise similar to the adult SPNs regarding clinicopathological characteristics [18].
The clinical presentation is nonspecific, as the patients, most frequently, solely complain of upper abdominal pain, bloating discomfort or, less frequently, an upper abdominal mass. Nevertheless, about one-third of the patients are asymptomatic and incidentally diagnosed on imaging. Likewise, no laboratorial abnormality is seen.
Rare presentations reported include acute rupture of an SPN, which is apparently more common in the pediatric population. It is associated with an acute abdomen presentation that usually follows trauma, or infrequently is spontaneous, possibly mimicking an acute appendicitis or an ovarian-related clinical condition. These conditions are ruled out by surgical exploration or imaging, which will rather show hemoperitoneum and a pancreatic mass, typically of large size (usually greater than 8 cm in diameter). The importance of this presentation relies on the presumption that this group of patients might have a higher incidence of metastatic disease, which prompts closer and longer follow-up [19].
Other rare presentations include: gastric outlet obstruction [20], splenic vein occlusion, and left-sided extrahepatic portal hypertension associated with gastric varices [21] and recurrent pancreatitis [22].
Despite being mostly a neoplasm of the pancreas, SPNs can also be extra-pancreatic, either derived from ectopic pancreatic tissue or from nonpancreatic tissue. Indeed, there are reports of SPNs located in the mesocolon, greater omentum, retroperitoneum, liver, stomach, duodenum and more recently, and being more frequently recognized as such, in the ovary and testis. These are clinically, histologically, and immunohistochemically similar to the pancreatic counterparts, so that this pathological diagnosis must be taken into consideration when dealing with extra-pancreatic masses that resemble pancreatic SPNs either on imaging or on histology [8, 23-25].
Diagnosis
Regardless of the clinical manifestations, a diagnosis of SPN is only considered after performing an imaging test, with a preoperative diagnosis obtained with endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) and/or an endoscopic ultrasound-guided fine-needle biopsy (FNB) with immunohistochemical staining (shown in Table 2 and 3) [26].
The neoplasm is most frequently a single mass [27], located in the body and tail of the pancreas [5, 14]. It is consistently a neoplasm of large size, with a mean diameter of 6 or 8 cm, according to the 2 largest case series available. However, it generally does not invade the surrounding structures nor does it cause biliary obstruction, even when located in the head of the pancreas [28]. Despite being a low-grade malignant neoplasm, it may rarely present with metastasis, commonly in the liver or infrequently in the peritoneum. When metastasis occur, these develop later in the course of the disease, usually 8-16 years after primary neoplasm resection [6, 26].
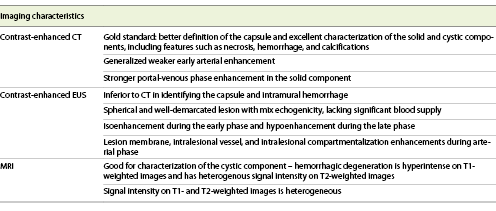
Computed tomography (CT) is the gold standard for the detection and evaluation of pancreatic masses, being the most frequently performed imaging modality in patients with SPNs [5, 14]. This neoplasm will appear on CT as a completely or incompletely encapsulated mass, unusually nonencapsulated, with both solid and cystic areas (shown in Fig. 1). Solid areas, sometimes appearing in the form of calcifications, are typically peripherical and correspond to pseudopapillary areas, whereas cystic areas are characteristically central and correspond to hemorrhagic degeneration. Contrast-enhanced CT (CECT) shows generalized weaker early arterial enhancement, but stronger portal-venous phase enhancement in the solid component [26, 29].
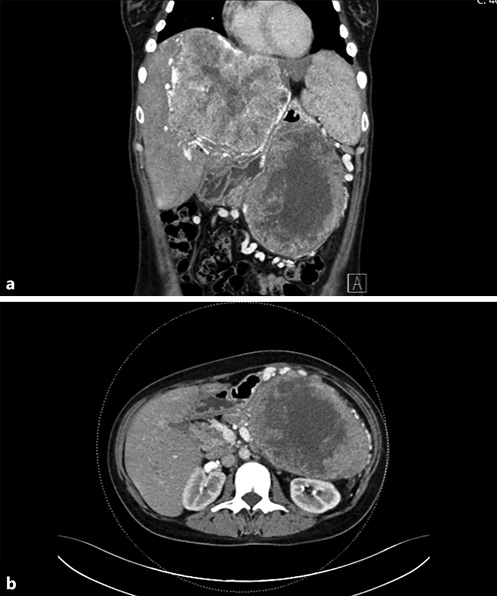
Fig. 1 Contrast-enhanced coronal (a) and axial (b) CT showing a large mass in the tail of the pancreas, with central hypodensity and heterogenous peripherical enhancement, associated with a giant hypervascular hepatic mass (a).
It has been discussed whether contrast-enhanced endoscopic ultrasonography (CEUS) can offer similar precision in diagnosing SPNs, compared to CECT, since it has several advantages, such as: (1) it provides real-time and dynamic imaging; (2) it does not use radiation; and (3) it can be employed in patients who are allergic to iodinated contrast media or have renal insufficiency [28]. It is becoming consensual that CEUS can, indeed, be a good alternative, as it can also show, a suggestive pattern of SPNs: (1) baseline endoscopic ultrasonography (EUS) reveals a spherical and well-demarcated lesion, with mix echogenicity (shown in Fig. 2), lacking significant blood supply; (2) CEUS shows isoenhancement during the early phase and hypoenhancement during the late phase plus lesion membrane, intralesional vessel, and intralesional compartmentalization enhancements during the arterial phase [26, 28, 29]. Nonetheless, CECT is superior to CEUS in identifying the capsule as well as intramural hemorrhage, which are the most important features for diagnosing SPN [26].
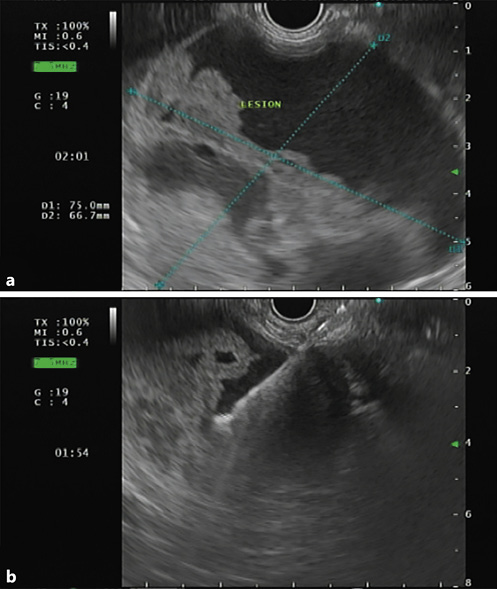
Fig. 2 a Endoscopic ultrasonography showing a large and well-demarcated lesion, located on the body and tail of the pancreas, with mixed echogenicity revealing solid and cystic components. b Fine-needle aspiration with a 19-gauge needle was performed for cytology and biochemical analysis.
Similarly, magnetic resonance imaging (MRI) can be a valuable exam, and can even be considered superior to CECT since, apart from the fact that it lacks radiation and is safer in those who suffer from contrast allergy or renal insufficiency, it better characterizes the cystic component of the lesion. MRI shows an encapsulated lesion with solid and cystic components plus hemorrhage without internal septation (shown in Fig. 3). In addition, the signal intensity on T1- and T2-weighted images is heterogeneous, owing to the presence of hemorrhagic degeneration, which is hyperintense on T1-weighted images and has heterogenous signal intensity on T2 weighted images.
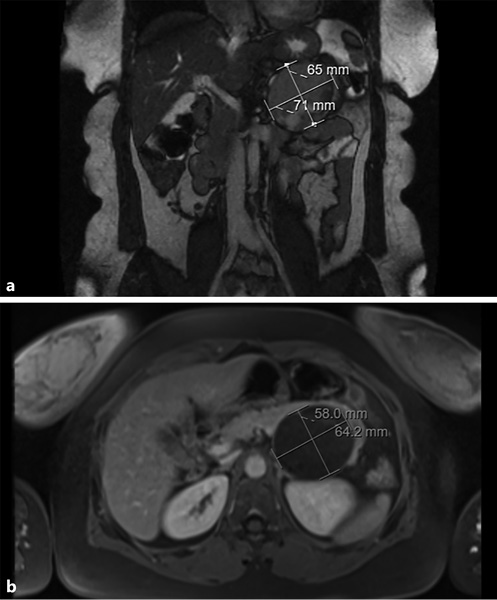
Fig. 3 T2-weighted (a) and T1-weighted (b) magnetic resonance images showing an encapsulated lesion in the body/tail of the pancreas, heterogeneously slightly hyperintense on the T2-weighted image and with low signal intensity on the T1-weighted image. Areas of hemorrhagic degeneration (low signal on the T2- and strong signal on the T1-weighted image) can also be seen.
Cytohistologic examination associated with immunohistochemistry is mandatory to confirm the diagnosis of SPN [30]. Endoscopic ultrasound-guided fine-needle aspiration (EUS-FA) with the aid of immunocytochemistry on cell block is the most frequently used procedure to accomplish this [31].
Provided that the correct preoperative diagnosis of SPN substantially relies on a cyto-histologic and immunohistochemical analysis, and taking into account the most recent studies comparing the role of FNB versus FNA in the diagnosis of pancreatic masses, EUS-FNB should be considered the gold standard option when tissue acquisition is required to obtain preoperative diagnosis of a SPN, since it provides better specimen adequacy with less needle passes [32-34].
FNA specimens are usually richly cellular, consisting of small discohesive and monomorphic cells surrounded by hemorrhagic debris and eventually multinucleated giant cells and/or foamy histiocytes. These cells display eosinophilic cytoplasm, containing occasionally hyaline globules and/or characteristic nuclear grooves [30].
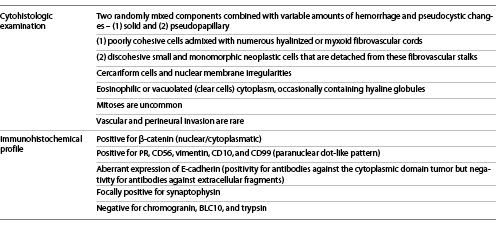
Histologic examination displays two randomly mixed components combined with variable amounts of hemorrhage and pseudocystic changes: (1) a solid component, composed of poorly cohesive cells admixed with numerous myxoid fibrovascular cords and (2) a pseudopapillary component, corresponding to discohesive small and monomorphic neoplastic cells that are detached from these fibrovascular stalks (shown in Fig. 4) [30]. The neoplastic cells’ cytoplasm is either eosinophilic or vacuolated (clear cells) and occasionally contains hyaline globules. Pathologists should look for additional characteristic features that, although not always present, are highly suggestive of SPN, such as cercariform cells [30, 35] and nuclear membrane irregularities, particularly nuclear grooves [36]. Mitoses are uncommon and vascular and perineural invasion is rare [30].
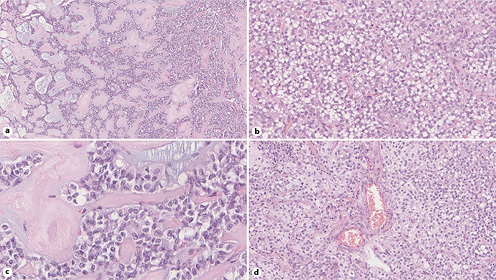
Fig. 4 Histopathology images from surgical specimen showing: a pseudopapillary pattern (hematoxylin and eosin [HE], original magnification, ×13) (a); solid tumor tissue featuring cells with clear cytoplasm (HE, original magnification, ×30) (b); nuclei with grooved membranes (HE, original magnification, ×35) (c); and hyaline globules between tumor cells (HE, original magnification, ×27) (d).
Moreover, WHO19 [3] emphasizes an SPN subtype - SPN with high-grade carcinoma, describing it as an “SPN with foci of high grade malignant transformation,” which is “characterized by diffuse sheets of cells with increased nuclear atypia, as well as abundant mitoses.” This subtype is clinically more aggressive [3].
Regarding the immunohistochemical profile, La Rosa and Bongiovanni [30] propose a panel for the routine pathology workup of suspected SPN, which includes the following markers: β-catenin, CD99, chromogranin, trypsin, BCL10, and E-cadherin. SPNs typically show nuclear/cytoplasmic immunoreactivity for β-catenin, very characteristic immunoreactivity for CD99 (dotlike paranuclear expression) and an aberrant expression of E-cadherin (positivity for antibodies against the cytoplasmic domain tumor but negativity for antibodies against extracellular fragments), while being negative for acinar cell markers (trypsin and BCL10) as well as for neuroendocrine marker chromogranin. Alternatively, we present the panel detailed in the paper by Bouça-Machado et al. [37] that illustrates a case series from our institution: PR (positivity), CD56 (positivity), vimentin (positivity), CD10 (positivity), β-catenin (nuclear positivity), synaptophysin (focal positivity), α1-antitrypsin (focal positivity), and chromogranin (negativity).
Differential Diagnosis
Main differential diagnosis of SPN include low-grade pancreatic neuroendocrine tumors (pNETs) in adults and pancreatoblastoma (PB) in children [10, 38, 39]. Pseudocyst and other pancreatic cystic neoplasms such as serous cystadenoma (SCA) and mucinous cystic neoplasm (MCN) should also be considered [9, 10, 39, 40].
pNET
pNET is the major entity when considering differential diagnosis of SPNs in the adult. Nonfunctioning cystic pNETs might clinically behave like SPNs. Despite being typically hypervascular on imaging examination, pNETs have hypovascular forms that can mimic SPNs [41, 42]. Shi et al. [41] have proposed a diagnostic model for differentiation of pNETs and SPNs, based on radiomics scores obtained from MRI’s derivations T2-weighted image (T2WI) and diffusion kurtosis imaging (DKI), age, and gender of the patients. Although successfully validated in this study, there is still need for more studies to validate this model, before it can be reliably applicable in clinical practice. Thus, presently the differential diagnosis cannot fully rely on clinical and imaging features. Furthermore, cytohistologic examination might as well be misleading, since a pseudopapillary pattern can resemble pseudorosettes characteristic of pNETs. However, a good cytologic examination will typically show other features consistent with a diagnosis, such as “salt and pepper” chromatin pattern for pNETs or nuclear membrane irregularities (nuclear grooves) for SPNs. Lastly, and more importantly, immunohistochemical examination will clarify and confirm the diagnosis: while pNETs are positive for cytokeratins (CK) 8 and 18 and neuroendocrine marker chromogranin, SPNs are not [10, 40]. In contrast to SPN, which portrays an excellent prognosis, even if presenting with metastasis or incomplete resection [30] and which is generally treated by neoplasm resection without lymph node dissection nor systemic therapy, pNETs have poorer prognosis (with metastasis: 5-year survival rate of 15-25%; if incomplete resection: survival rate of 35-75%) and must be approached more aggressively, with lymph node dissections and systemic therapy if advanced locoregional or metastatic disease is present [41]. Thereby, the differential of these entities is crucial to the planning and decision-making processes.
Pancreatoblastoma
SPNs and PBs are the most frequent neoplasms of the pancreas in children [43]. Despite being clinically identical to SPNs, PBs cytologic examination usually leaves no doubts, since these have a distinctive organoid pattern with characteristic squamoid corpuscles and acinar cells containing zymogen granules [43]. Zhaoxia Yang et al. [38] came up with clinical and imaging features that could be useful in differentiating these entities. Features that support the diagnosis of PB are age <5 years old, elevated alpha-fetoprotein level, not well demarcated tumor margins, size >6.1 cm, tumoral calcification, peripancreatic vessel invasion, and metastasis at the time of diagnosis. Nevertheless, cytologic examination is still imperative for an accurate preoperative differential diagnosis, which is of great importance since PB is substantially more susceptible to recurrence and metastasis than SPN.
Pseudocyst
SPNs with extensive cystic degeneration can mimic pseudocysts. However, patient’s history, imaging and eventually cytologic and cystic fluid analysis will unveil the diagnosis. Pseudocysts mostly arise following episodes of acute pancreatitis, particularly if superimposed on chronic alcoholic pancreatitis, and are diagnosed following imaging examination showing a thick-walled, rounded and fluid-filled mass in the pancreas. If performed, (1) cytologic examination will display debris, blood, inflammatory cells and occasionally yellow pigment or crystals; (2) fluid analysis will have elevated concentration of amylase; and (3) histologic examination will show a pancreatic cystic lesion with no epithelial lining, lined instead by fibrin and granulation tissue.
Other Cystic Neoplasms
Similar to SPNs, SCAs and MCNs are cystic lesions most often incidentally diagnosed in female patients [40].
SCA is typically diagnosed in the 6th-7th decade of life and is characterized: (1) on imaging examination, for displaying central, stellate calcifications; (2) on fluid analysis, for having low levels of CEA; (3) histologically for displaying multiple cysts lined by glycogen-rich cuboidal cells and cells with round nuclei and remarkably uniform homogenous chromatin; (4) immunohistochemically for staining positive for cytokeratins and negative for CEA [40].
MCNs are diagnosed almost exclusively in middle-aged women and are characterized: (1) on imaging examination, for showing as a multilocular/septated, occasionally with peripheral calcifications neoplasm; (2) on fluid analysis for its thickness and high CEA levels; (3) cytologically for focal clusters of mucin-containing epithelium; (4) histologically for displaying cysts filled with thick mucin and lined by a columnar mucin-producing epithelium associated with a densely cellular ovarian-type stroma; and (5) immunohistochemically for progesterone and estrogen receptor-positive stain in the ovarian stroma [40].
Treatment
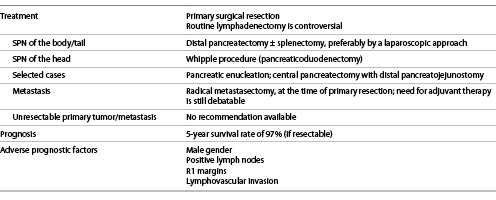
Primary surgical resection is the mainstay of treatment for SPNs (shown in Table 4). The procedure of choice will depend on the localization of the tumor. SPN of the body or tail of the pancreas should be resected by distal pancreatectomy, preferably using a laparoscopic approach, with spleen preservation whenever feasible (which can only be considered after exclusion of splenic vasculature and hilar involvement). On the other hand, SPN of the head of the pancreas should be managed by a Whipple procedure (pancreaticoduodenectomy), using an open approach, with pylorus preservation whenever possible, since it reduces the postoperative morbidity by decreasing the risk of dumping syndrome and diarrhea [26, 29, 44].
Since it is a “low grade malignant tumor,” when the tumor is localized, there is also the possibility to employ more conservative and parenchyma-preserving techniques, such as pancreatic enucleation and central pancreatectomy with distal pancreatojejunostomy, thus preserving pancreatic exocrine and endocrine functions. Enucleation can be an alternative approach when the tumor is smaller than 2 cm, its distance to the main duct is more than 2-3 mm and especially when it is localized in the head of the pancreas. Furthermore, it can be conducted laparoscopically. Similarly, central pancreatectomy, by open approach, can be an alternative for tumors localized in the neck of the pancreas and smaller than 3-5 cm. Despite their benefits in maintaining pancreatic functions, these techniques are associated with an increased incidence of pancreatic fistula and a higher postoperative morbidity [45]. Therefore, parenchyma-preserving techniques should be, then, only pursued in young and fit patients who can tolerate the associated higher postoperative morbidity. Also, it could be debatable whether parenchyma-preserving technique indications should not be less strict (for instance, considering safety margins) in the pediatric population [46].
The need for lymphadenectomy in these patients remains a controversial issue: a few authors claim that lymphatic dissections should be employed routinely, but many authors advise against it [16, 26]. However, when suspicious lymph nodes are found intraoperatively, it is undoubtedly consensual that these should be removed.
In the cases where patients present with liver metastasis, radical metastasectomy, at the time of primary resection, should always be attempted if the lesion is deemed resectable with at least 1 cm margin. In these situations, generally there is no need for systemic therapy, although the benefits of its use in metastatic resectable tumors are still debatable. In the rarer cases of peritoneal metastasis, these can be approached by complete cytoreductive surgery (CCRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) with irinotecan and oxaliplatin [26, 29].
If metastatic lesions are not deemed suitable for resection, there is still no consensual recommendation. There are case reports describing successful attempts using adjuvant therapy with: (1) yttrium-90 selective internal radiation therapy (Y-90 SIRT); (2) chemosaturation with percutaneous hepatic perfusions of melphalan; (3) a combination of the tyrosine kinase inhibitor sunitinib and hepatic artery embolization; (4) radiofrequency ablation; (5) liver transplant [26, 29, 47-50].
Lastly, attempts to treat unresectable SPNs with adjuvant chemoradiotherapy (in one report comprising gemcitabine) have been reported in the literature, but there is still lack of evidence to make a recommendation for these situations [26, 29, 50].
Special Consideration - SPN in Pregnant Women
SPN diagnosis during pregnancy is rare. Santos et al. [13] summarize all case reports of SPN during pregnancy described in the literature. We have calculated the mean tumor size for these cases (11.9 ± 3.6 cm). Although the small population size does not allow statistical inferences with optimal external validity, it seems that SPNs tend to be larger in pregnant women, which could make one think these are more likely to present with symptoms. However, as SPN presentation is highly nonspecific and understandably confoundable with pregnancy common symptoms (abdominal discomfort, nausea, vomiting), diagnosis can be even more challenging. Also, during diagnosis workup, clinicians must think differently when deciding which image modality best suits these patients since the safety of the fetus must be on the top of their priorities. Consequently, modalities without emission of ionizing radiation and without any known adverse fetal effect, such as EUS and MRI, are the gold-standard [13, 51, 52].
Currently, there is no consensus or guidelines for SPN management in pregnant women. Optimal treatment timing is the core of the discussion, and the only agreement on the matter is that it should be always decided on a case by case basis: clinicians can opt for close surveillance during pregnancy and safely postpone the resection to the postpartum period [52] or, on the other hand, can decide to go ahead with surgical intervention regardless of the gestational age, especially if emergent intervention is required (e.g., due to tumor rupture). It is noteworthy that case reports describing surgical intervention during pregnancy showed no harm to fetal development [13, 51, 52].
Prognosis
Overall, the prognosis is remarkably good, even in the presence of metastasis, with an overall 5-year survival rate of about 97%. Unresectable disease is associated, on the other hand, with a worse but still fair prognosis, since SPNs are slow-growing neoplasms, with a calculated double time of 765 days [53]. Recurrence after radical resection can occur, though, in 2-10% of the cases [5, 14, 54].
The time of follow-up and the conditions requiring closer follow-up are still topics of debate. Nevertheless, an annual surveillance has been suggested for at least 5 years [55].
Several studies have been designed to come up with tumor markers that could predict recurrence or patient outcome. Sex, age, tumor size, surgical margins, perineural invasion, angioinvasion, deep infiltration of surrounding structures, and Ki-67 proliferative index have been suggested, but published results are not consensual [30].
Furthermore, it is noteworthy that the latest systematic review on this topic concluded that features such as male gender (odds ratio [OR] 1.960, 95% confidence interval [95% CI] 1.010-3.805, p = 0.047), positive lymph nodes (OR 11.918, 95% CI 3.798-37.292, p not available), R1 margins (OR 11.132, 95% CI 4.342-28.541, p not available), and lymphovascular invasion (OR 5.504, 95% CI 2.461-12.311, p not available) are associated with statistically significant higher odds for recurrence. Thereby, until better consensus, it is advisable to adopt closer follow-up programs in patients presenting with these features [56].
More recently, microRNA expression pattern using a panel of six microRNAs [57] and preoperative neutrophil-to-lymphocyte ratio [58] were suggested to be potential markers, but there is still lack of evidence.
Conclusion
Key Messages
Clinicians should be aware of this entity when approaching a young female presenting with a pancreatic mass.
Cytohistologic examination associated with immunohistochemistry is required to confirm the diagnosis of SPN.
EUS-FNB should replace EUS-FNA since it is more accurate for preoperative diagnosis of pancreatic masses.
Primary surgical resection is the mainstay of treatment for these neoplasms.
More evidence about the need for lymphadenectomy and the approach to unresectable disease is needed.
The prognosis is remarkably good, with a 5-year survival rate of about 97%.
More high-quality studies about poor outcome predictors are needed