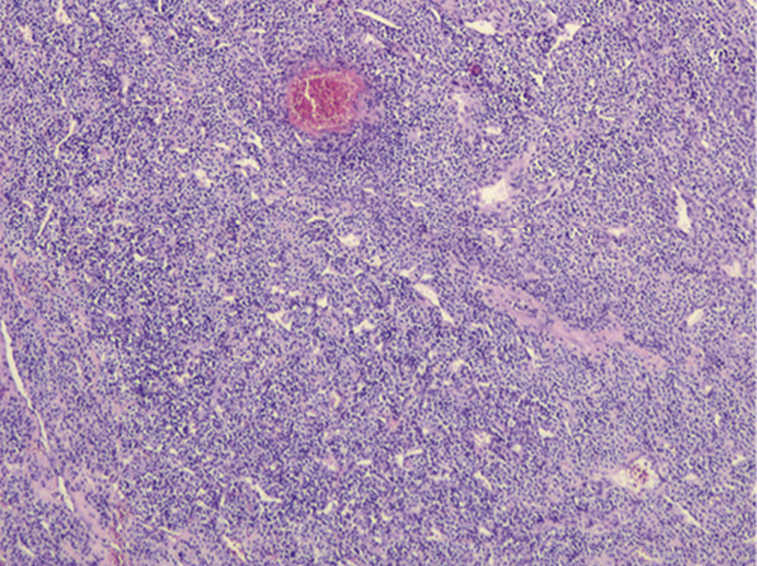
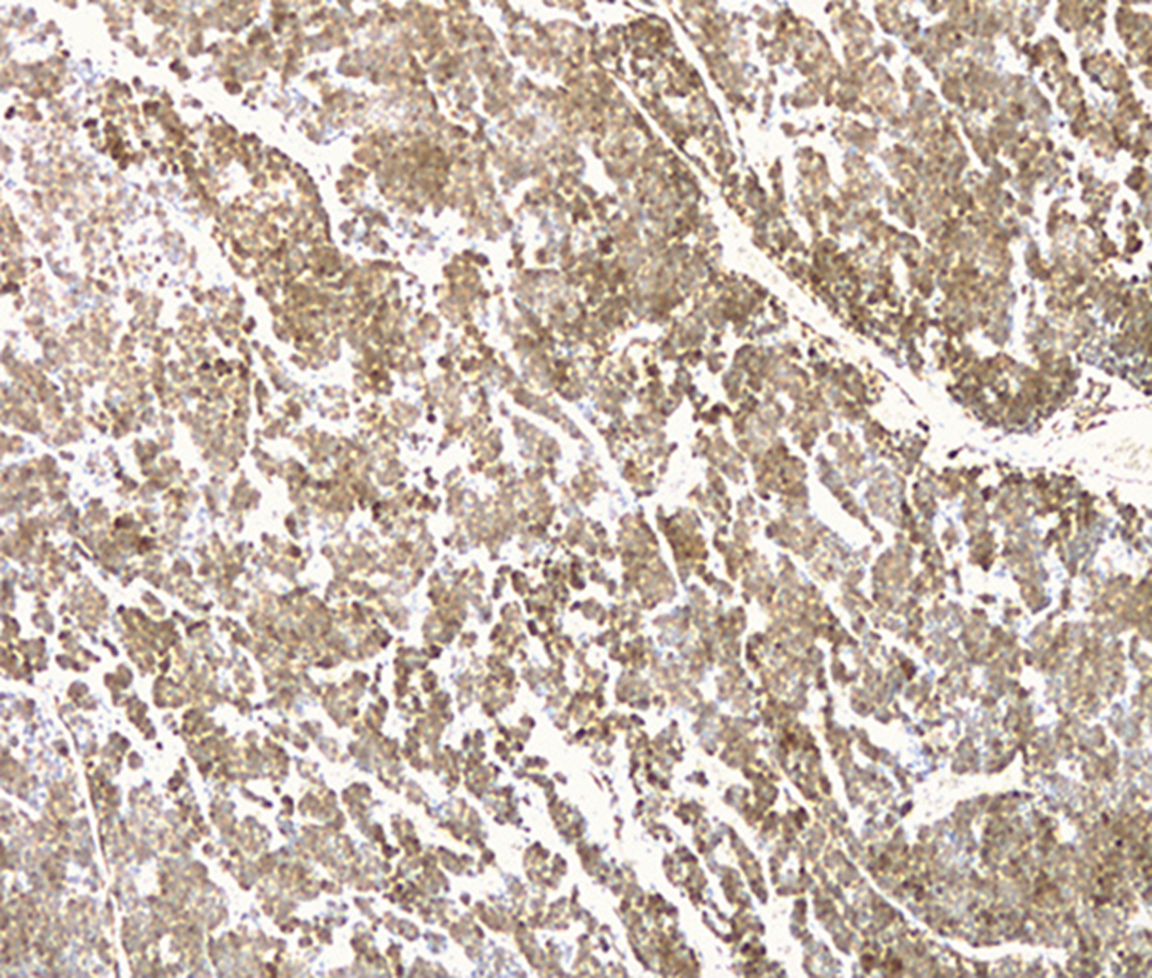
A 37-year-old leucodermic woman with no relevant past history presented with 1-month history of new-onset epigastric pain and bloating. She denied any other symptoms. She underwent an esophagogastroduodenoscopy that revealed a 25-mm subepithelial lesion in the gastric antrum (Fig. 1). Tunnel biopsies were done and were unrevealing. She underwent an endoscopic ultrasound (EUS) that showed a 25-mm hypoechogenic subepithelial lesion originating in the muscularis propria (Fig. 2). EUS-guided fine-needle aspiration (FNA) with a 25-G needle (Boston Scientific®) was performed (2× passes using suction technique), revealing cell nests without atypia, that were focally positive for smooth-muscle actin and synaptophysin, and negative for chromogranin and CD117. KIT and PDGFRA mutations were also negative. Unfortunately, the sample was inadequate for further study. A staging CT was performed excluding distant metastasis. Since investigations thus far were inadequate to exclude a malignant process, she underwent laparoscopic-wedge gastrectomy. Histopathology revealed a solid, epithelioid, and richly vascular tumor without cellular atypia (Fig. 3). Immunohistochemistry was similar to that previously performed and complemented with positive calponin (Fig. 4) and negative cytokeratin AE1/AE3 (Fig. 5), making the diagnosis of a glomus tumor.
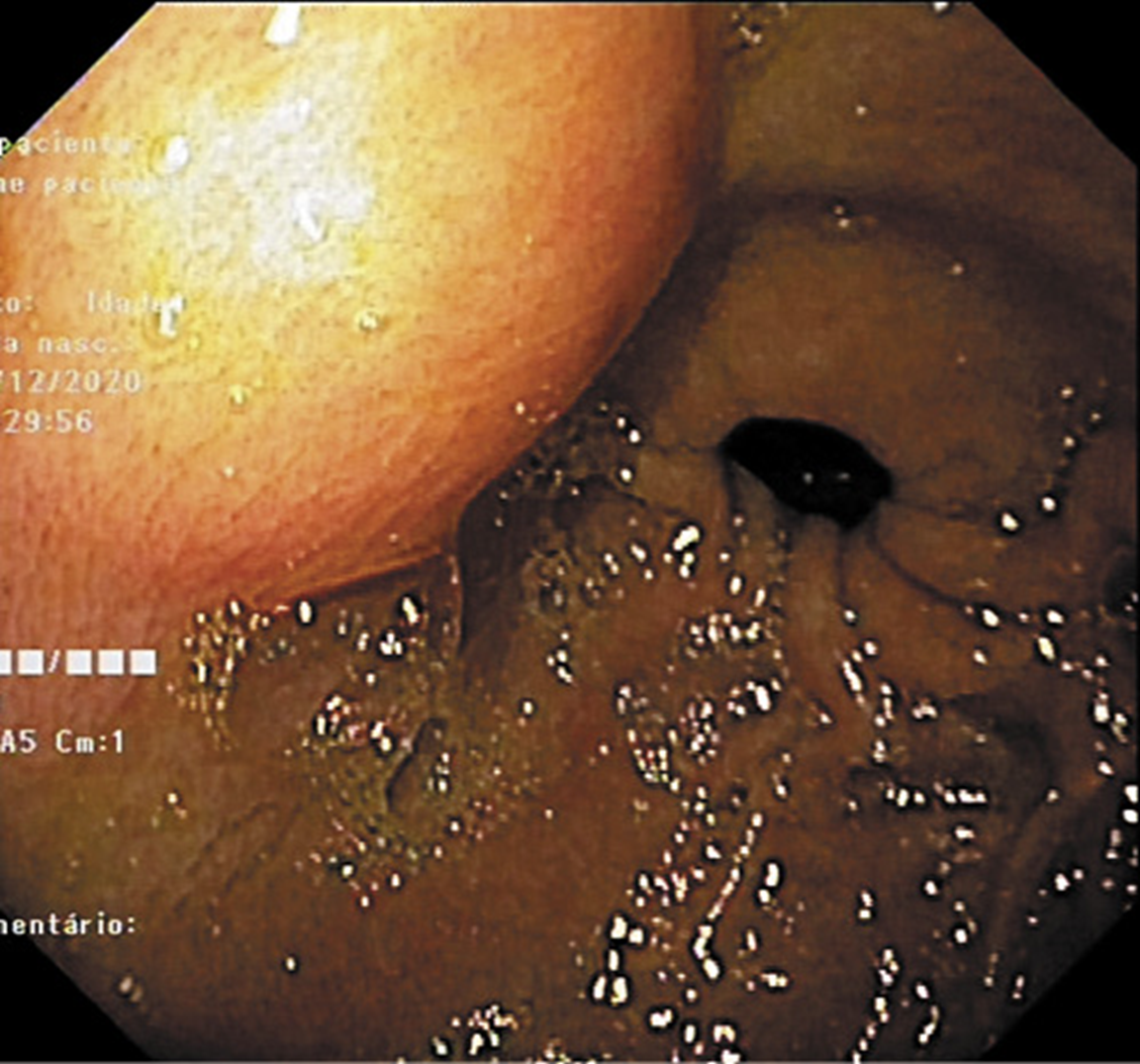
Fig. 1: Esophagogastroduodenoscopy revealed a 25-mm subepithelial gastric lesion in the gastric antrum.
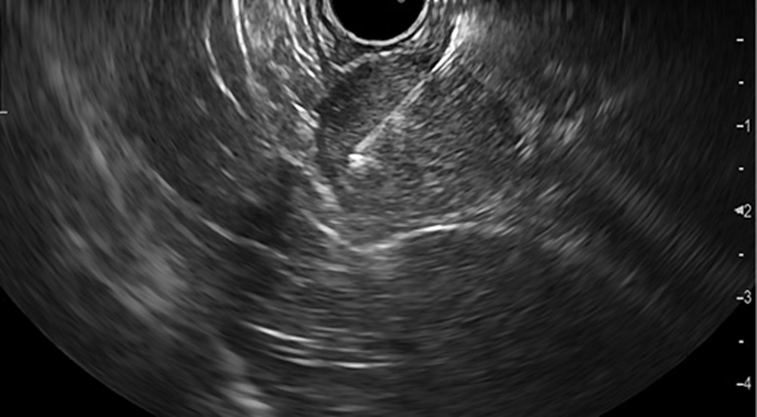
Fig. 2: EUS showing a 25-mm hypoechogenic subepithelial gastric lesion originating in the muscularis propria.
Glomus tumors are rare mesenchymal neoplasms that originate in modified smooth-muscle cells (1-3). Despite being highly vascularized, they are most often benign (3). They are frequently found in the skin and subcutaneous tissue, although they can also be located in the gastrointestinal tract, most commonly in the stomach (2, 3). Gastric glomus tumors are usually located in the antrum (2, 3). Clinical symptoms are nonspecific, ranging from dyspepsia to gastrointestinal bleeding (3). Endoscopically, they appear as a smooth submucosal lesion, usually 2-3 cm in size, and present as an ulcerated submucosal lesion in roughly 45% of cases (3). On EUS, they typically originate from the muscularis propria (4th echo layer) but can also be found in the submucosal or mucosal layers (1). Additionally, they can present either as hyperechoic or hypoechoic, usually have internal hyperechoic foci, and present a prominent Doppler signal consistent with the vascular nature of these tumors (1, 2). However, it can be difficult to distinguish them from other subepithelial lesions (e.g., gastrointestinal stromal and neuroendocrine tumors) since they do not exhibit specific distinguishing features on EUS (2). EUS-FNA is an effective method to obtain cytological specimens of gastric submucosal neoplasms, allowing for an adequate histopathological evaluation when using the cell-block technique. However, to ensure an adequate diagnosis of a gastric submucosal lesion, it is important to perform an immunohistochemical study on the cell block (4). Gastric glomus tumors are typically positive for actin, calponin, and vimentin, and negative for CD117, CD20, and CD45, chromogranin A, desmin, and S-100 protein, allowing the distinction of these tumors in the differential diagnosis (3). Since malignant transformation has been described, a complete resection of the lesion is mandatory for definitive treatment (2, 3). The surgical approach depends on the location and size of the tumor. Zhang et al. (2) reported an effective endoscopic approach through endoscopic submucosal dissection technique. However, due to its infrequency, there are insufficient data to establish guidelines regarding management, including the need for postoperative surveillance.
This case denotes the diagnostic challenge and the not-so-linear diagnostic evaluation when facing a gastric subepithelial lesion. The key point is that gastroenterologists should be aware of this rare, difficult-to-diagnose, and potentially malignant entity in the differential diagnosis of subepithelial lesions, especially when present in the stomach.