Introduction
Upper gastrointestinal bleeding (UGIB) can be divided into variceal and nonvariceal (NVUGIB). The incidence of NVUGIB is 80-150/100,000, and the mortality rate is 2-15% [1]. Patients may have hematemesis or melena, although hematochezia can also occur in the context of major bleeding, associated with hemodynamic instability [2].
The initial approach to these patients should focus primarily on adequate fluid replacement and blood transfusion if needed [3]. Secondly, upper gastrointestinal (GI) endoscopy is the preferred diagnostic tool to find the bleeding source, and it also allows therapeutic approaches [4]. The most common identified cause of NVUGIB is peptic ulcer disease, followed by erosive esophagitis, Dieulafoy lesion, Mallory-Weiss syndrome, gastric antral vascular ectasia, and neoplasms [3, 5].
Management of these patients in the urgent care setting requires an effective stratification of the risk underlying each case. Two of the most commonly used scoring systems are the Rockall score (RS) and the Glasgow-Blatchford score (GBS) [6, 7]. Although the predictive value of tools has been extensively validated, their clinical effectiveness remains unclear [7]. Their accuracy and optimum thresholds to identify low-risk and high-risk patients have also been poorly studied [8].
RS was designed do predict mortality [9], and its components include age, shock severity, presence of comorbidities, as well as endoscopic findings, ranging from 0 to 11 [9, 10]. A “pre-endoscopy” or “clinical” RS (ranges from 0 to 7) can be calculated before endoscopy and may be useful for initial patient assessment, although its predictive ability might decrease [11].
GBS was designed to identify which patients need endoscopic assessment [9] and it is commonly used to identify low-risk patients suitable for outpatient management [12]. This tool ranges from 0 to 23 and includes clinical presentation (melena, syncope, systolic blood pressure, and heart rate), laboratory results (blood urea and hemoglobin), and the presence of comorbidities (hepatic/cardiac disease), excluding endoscopic findings [13].
The present study aims to evaluate the prognostic value of these scores in patients admitted with acute NVUGIB at Centro Hospitalar Universitário do Porto (CHUP) by calculating pre-endoscopic RS, complete RS, and GBS simultaneously for each patient and comparing different outcomes according to these scores. This institution provides an emergency endoscopy service, 24 h a day, 7 days a week. This out-of-hours model of care was created to make the most out of medical resources available from 6 gastroenterology departments in only 1 facility from 8 p.m. to 8 a.m. This organized strategy implies an accurate risk stratification in order to avoid underuse or overuse of medical care. The main goal is to analyze the scores’ ability to predict clinical outcomes, such as mortality, rebleeding, need for blood transfusion, need for endoscopic intervention, surgery requirement, as well as need for hospitalization in the Intermediate Care Unit (IMCU) or the Intensive Care Unit (ICU). Secondarily, this study intends to evaluate the appropriateness of patients’ transfers from other institutions according to their risk scores in order to understand if we deliver the best care possible to every patient in an equitable and efficient manner.
Materials and Methods
The present study was retrospectively conducted at CHUP and included patients admitted to the Emergency Department with acute manifestations of NVUGIB that underwent upper GI endoscopy between January 2016 and December 2018. The following information was collected from the clinical records of each patient: demographic data (age and gender), symptoms and signs on admission, comorbidities, use of anticoagulant/anti-platelet drugs, laboratory results (hemoglobin, hematocrit, platelets, creatine, urea, albumin, bilirubin, and sodium), endoscopic findings and therapeutic approach, as well as subsequent clinical outcomes, such as mortality, rebleeding, requirement for blood transfusion, need for additional endoscopic intervention, need for surgery, as well as demand for hospitalization in the IMCU/ICU. RS (pre-endoscopic and complete) and GBS were calculated. The exclusion criteria were: UGIB in already hospitalized patients and insufficient clinical and endoscopic information.
The Charlson Comorbidity Index (CCI) was the chosen tool to evaluate the magnitude of the patient’s comorbidities, ranging from 0 to 23. It is a well-validated score for measuring comorbidity in many different contexts, including UGIB.
Categorical variables were compared using the χ2 test. Mann-Whitney U test was used to compare means of continuous independent variables. Receiver operating characteristic (ROC) curves were used to identify the cutoff point that maximizes the sum between sensitivity and specificity of each score to predict the different clinical outcomes. The areas under the curves (AUC) were calculated to estimate the predictive power of the different scoring systems. An AUC higher than 0.7 was used to consider good performance. A p value of 0.05 or less was considered statistically significant. All the data collection and statistical analysis was done using the IBM SPSS Statistics version 26.
Mortality and rebleeding were defined as events that occurred during the index hospitalization. Endoscopic procedures available were adrenalin injection, mechanical treatment (clips, band ligation, or over-the scope), sclerotherapy, and/or thermal therapy (bipolar electrocoagulation or heater probe).
Results
Baseline Characteristics
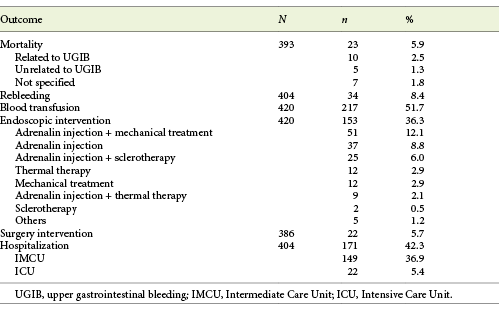
A total of 545 patients presented with endoscopically confirmed NVUGIB at the Emergency Department. Four hundred and twenty patients were included, and 125 (22.94%) cases were excluded. Exclusion criteria were: already hospitalized patients and patients with incomplete clinical information.
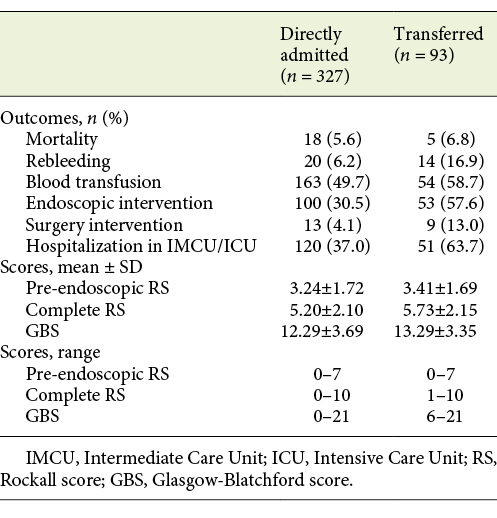
The mean age was 68.27 ± 16.5 years and the ratio of men to women was 2.92:1. Regarding patients’ comorbidities, the CCI mean was 4.74 ± 3.16, with the most common comorbidities being congestive heart failure (26.2% of all the patients) and diabetes mellitus (25.7%). Most of the patients included in this study were direct admissions to CHUP (77.9%), while a smaller percentage (22.1%) was transferred from other hospitals (Table 1). Clinical presentation at admission was diverse, as shown in Table 1.
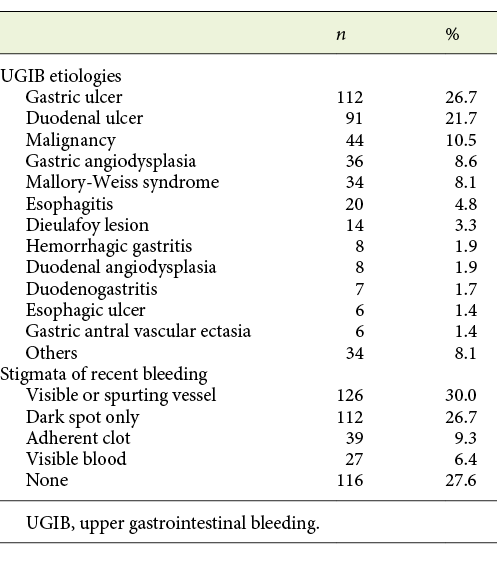
The most common sources of bleeding were peptic ulcer (48.4%) and malignancy (10.5%). In regard to endoscopic stigmata of recent bleeding, 30% presented a visible or spurting vessel, 27.6% had no stigmata, 26.7% had dark spot only, and a minority of patients presented adherent clot (9.3%) or visible blood in the upper GI tract (6.4%) (Table 2). Forrest classification was used to classify peptic ulcer cases found: 7.8% were Forrest Ia, 7.3% Ib, 20.5% IIa, 9.8% IIb, 27.3% IIc, and 27.3% III.
Table 2: Upper gastrointestinal bleeding etiologies and endoscopic stigmata of recent bleeding (n = 420)
Mean pre-endoscopic RS was 3.35 ± 1.71, mean complete RS was 5.32 ± 2.11, and mean GBS was 12.15 ± 3.64. Overall, there were 23 (5.9%) in-hospital deaths reported, and the rate of rebleeding was 8.4%. Over half of the patients required blood transfusion (51.7%). A total of 153 patients (36.3%) received endoscopic treatment, 22 (5.7%) required surgery, and 171 (42.3%) required IMCU or ICU care (Table 3). Mean length of hospital stay was 11.31 ± 14.08 days.
Comparison of Scores’ Ability to Predict Outcomes
Mortality
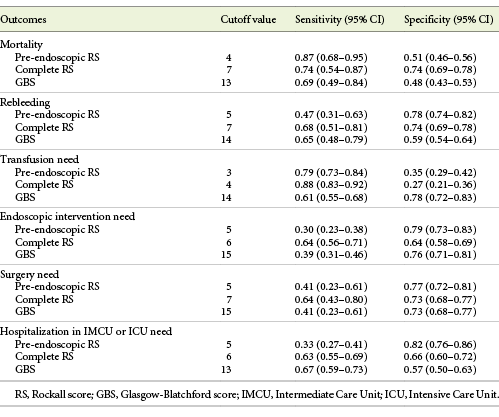
Patients who died had higher complete RS than the ones who survived (6.91 ± 1.76 vs. 5.15 ± 2.08, p < 0.001). This was also confirmed for pre-endoscopic RS (4.43 ± 1.27 vs. 3.25 ± 1.71, p = 0.001), although it was not true for GBS (13.52 ± 4.65 vs. 12.40 ± 13.51, p = 0.108). According to ROC curve analysis, complete RS showed the best discriminative ability in predicting mortality with an AUC of 0.756 (CI 0.62-0.81, p < 0.001). Pre-endoscopic RS also showed good performance with an AUC of 0.711 (CI 0.66-0.86, p = 0.001). GBS had no discriminative ability in this outcome (AUC 0.599, CI 0.459-0.740, p = 0.110) (Fig. 1a; Table 4).
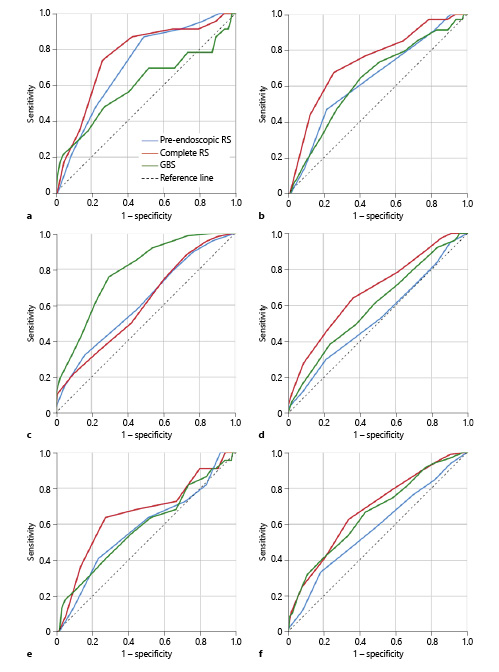
Fig. 1: ROC curves comparing scores for mortality (AUC: pre-endoscopic RS = 0.711, complete RS = 0.756, GBS = 0.599) (a), rebleeding (AUC: pre-endoscopic RS = 0.635, complete RS = 0.735, GBS = 0.631) (b), transfusion need (AUC: pre-endoscopic RS = 0.617, complete RS = 0.606, GBS = 0.785) (c), endoscopic treatment need (AUC: pre-endoscopic RS = 0.533, complete RS = 0.677, GBS = 0.597) (d), surgery need (AUC: pre-endoscopic RS = 0.578, complete RS = 0.658, GBS = 0.585) (e), hospitalization need (AUC: pre-endoscopic RS = 0.574, complete RS = 0.677, GBS = 0.657) (f).
Rebleeding
All scores were higher in patients that rebled (complete RS: 6.82 ± 1.78 vs. 5.13 ± 2.09, p < 0.001, pre-endoscopic RS: 4.03 ± 1.51 vs. 3.26 ± 1.73, p = 0.008, and GBS: 13.79 ± 3.37 vs. 12.39 ± 3.59, p = 0.011). ROC curve analyses showed that the complete RS had discriminative capability in predicting rebleeding with an AUC of 0.735 (CI 0.647-0.823, p < 0.001). Both pre-endoscopic RS and GBS did not perform well with AUCs of 0.635 (CI 0.539-0.731, p = 0.009) and 0.631 (CI 0.534-0.728, p = 0.011), respectively (Fig. 1b; Table 4).
Blood Transfusion
Patients who required blood transfusion presented with higher scores (complete RS: 5.76 ± 2.00 vs. 4.84 ± 2.13, p < 0.001, pre-endoscopic RS: 3.72 ± 1.65 vs. 2.96 ± 1.70, p < 0.001, and GBS: 14.26 ± 2.71 vs. 10.64 ± 3.57, p < 0.001). According to ROC analyses, GBS performed well with an AUC of 0.785 (CI 0.742-0.828, p < 0.001). Pre-endoscopic RS had an AUC of 0.617 (CI 0.564-0.670, p < 0.001) and complete RS had an AUC of 0.606 (CI 0.552-0.659, p < 0.001) (Fig. 1c; Table 4).
Endoscopic Intervention
Complete RS (6.18 ± 1.96 vs. 4.82 ± 2.04, p < 0.001) and GBS (13.35 ± 3.39 vs. 12.03 ± 3.69, p = 0.001) were both higher in patients requiring endoscopic intervention. This was not observed for pre-endoscopic RS (3.50 ± 1.76 vs. 3.27 ± 1.68, p = 0.253). According to ROC curve analyses, none of the scores showed good performance in predicting this need: complete RS with an AUC of 0.677 (CI 0.624-0.730, p < 0.001), GBS with an AUC of 0.597 (CI 0.541-0.654, p = 0.001), and pre-endoscopic RS with an AUC of 0.533 (CI 0.475-0.591, p = 0.262) (Fig. 1d; Table 4).
Surgery
Patients that underwent surgery presented higher complete RS (6.32 ± 2.12 vs. 5.21 ± 2.07, p = 0.12). This was not true for both of the other scores (pre-endoscopic RS: 3.73 ± 1.72 vs. 3.32 ± 1.71, p = 0.210, and GBS: 13.50 ± 3.95 vs. 12.42 ± 3.57, p = 0.177). For the prediction of this outcome, there was also no evidence of good performance of any of the scores, with complete RS showing an AUC of 0.658 (CI 0.529-0.787, p = 0.013), pre-endoscopic RS an AUC of 0.578 (CI 0.449-0.707, p = 0.218), and GBS an AUC of 0.585 (CI 0.453-0.718, p = 0.179) (Fig. 1e; Table 4).
IMCU or ICU Hospitalization
Lastly, patients that required hospitalization in these wards presented higher complete RS (6.06 ± 1.95 vs. 4.73 ± 2.05, p < 0.001) and GBS (13.66 ± 3.38 vs. 11.61 ± 3.63, p < 0.001), although this was not confirmed by pre-endoscopic RS (3.60 ± 1.74 vs. 3.18 ± 1.68, p = 0.010). According to ROC curve analysis, there was no statistical significance in the prediction of hospitalization for complete RS (AUC 0.677, CI 0.625-0.730, p < 0.001), GBS (AUC 0.657, CI 0.604-0.711, p = 0.001), or pre-endoscopic RS (AUC 0.574, CI 0.517-0.630, p = 0.011) (Fig. 1f; Table 4).
Transferred versus Directly Admitted Patients
Transferred and nontransferred groups presented similar mean ages (68.32 ± 17.54 vs. 68.26 ± 15.88 years, p = 0.977) and similar gender distribution (men vs. women: 71.7 vs. 75.3%, p = 0.488). Regarding patients’ comorbidities, the CCI mean was also identical between the 2 groups (4.13 ± 2.72 vs. 4.91 ± 3.26, p = 0.099).
There was no statistical difference between transferred and nontransferred patients regarding mean pre-endoscopic RS (3.41 vs. 3.34, p = 0.692) and GBS (13.29 vs. 12.29, p = 0.056), while mean complete RS (5.73 vs. 5.20, p = 0.026) was higher in transferred patients. Although the mean GBS score did not vary significantly between the 2 groups, only patients with a score of 6 or more were transferred to this facility, whilst directly admitted patients presented scores of 0 or more. Regarding pre-endoscopic RS and complete RS, the range of scores for transferred and nontransferred patients were very similar, as seen in Table 5.
Table 5: Differences in scores and outcomes between directly admitted patients and transferred patients
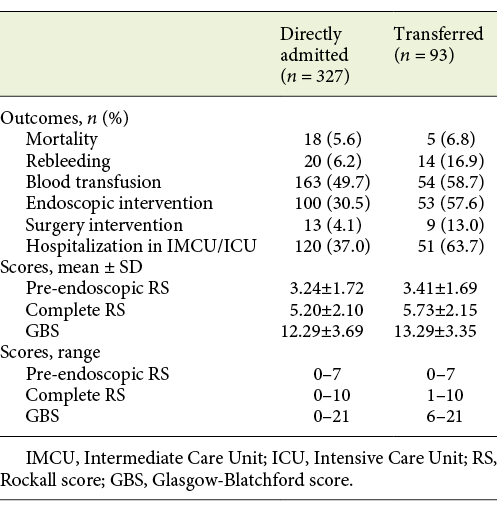
Some outcomes’ rates were significantly higher in transferred patients: rebleeding (16.9 vs. 6.2%, p = 0.002), endoscopic treatment (57.6 vs. 30.5%, p < 0.001), surgery (13.0 vs. 4.1%, p = 0.004), and hospitalization in the IMCU/ICU (63.7 vs. 37.0%, p < 0.001). Mortality (6.8 vs. 5.6%, p = 0.713) and transfusion (58.7 vs. 49.7%, p = 0.127) rates between transferred and nontransferred patients were not statistically different (Table 5).
There were no deaths reported with pre-endoscopic RS of 0, complete RS of 0 or 1, and GBS of 0-3. In the transferred group, there were 5 deaths reported, and the patients presented pre-endoscopic RS ≥4, complete RS ≥7, and GBS ≥11. There were no records of rebleeding in patients with pre-endoscopic RS of 0, complete RS of 0 or 1, and GBS of 0-3. Concerning transferred patients, 14 rebleeding episodes were described with pre-endoscopic RS ≥1, complete RS ≥4, and GBS ≥10. There was no need for transfusion in any patients with complete RS of 0 or with GBS of 0-6, but there were cases of transfusion in all of the pre-endoscopic RS scores. Transferred patients that required blood transfusion presented pre-endoscopic RS ≥0, complete RS ≥1, and GBS ≥7. No endoscopic treatment was required in patients with complete RS of 0-2 and GBS of 0-4, although there were cases of endoscopic therapy in every score of pre-endoscopic RS. All of the transferred patients that received endoscopic therapy had pre-endoscopic RS ≥0, complete RS ≥2, and GBS ≥6. There was no need for surgery with pre-endoscopic RS of 0, complete RS of 0 or 1, and GBS of 0-3. Only 9 of the transferred patients required surgery, all of them with pre-endoscopic RS ≥0, complete RS ≥4, and GBS ≥11. There was no need for close care in patients with complete RS of 0 and GBS of 0-3. This was required in patients with every score of pre-endoscopic RS. Transferred patients that required this type of hospitalization presented pre-endoscopic RS ≥0, complete RS ≥1, and GBS ≥6.
Discussion/Conclusion
Of a total of 420 patients, 5.9% died, 8.4% rebled, 51.7% received blood transfusion, 36.3% received endoscopic therapy, 5.7% had surgery, and 42.3% required hospitalization in the IMCU/ICU. Regarding mortality prediction, both complete RS and pre-endoscopic RS showed good performance. In the prediction of rebleeding, only complete RS had discriminative ability. GBS had good performance in the prediction of transfusion. No score showed discriminative capability in the prediction of other outcomes. Transferred and nontransferred patients had similar pre-endoscopic RS and GBS. Only patients with GBS ≥6 were transferred to our facility. There were no adverse outcomes recorded in any group when GBS ≤3.
The main goal of this study was to analyze the scores’ ability to predict clinical outcomes, such as mortality, rebleeding, requirement for blood transfusion, need for endoscopic intervention, need for surgery, as well as demand for hospitalization in the IMCU or ICU.
A mortality rate of 5.9% calculated in this study shows the importance of identifying patients at low and high risk of death due to NVGIB. The present study concluded that complete RS has the most discriminative value in predicting mortality, followed by pre-endoscopic RS, while GBS showed poor performance, which is comparable to most studies analyzed [7, 8, 14]. Unlike our study, some articles [14-16] report GBS with AUCs higher than 0.7, although it still does not perform as well as the others. Chandnani et al. [17] did not include pre-endoscopic RS in their study but still found complete RS to have good performance and GBS to have poor performance. Regarding cutoff values, scores of 5 [8, 15, 17] and 7 [14, 16] were the most common ones described for complete RS, which is in agreement with the cutoff of 7 found in our study. The most frequent ones reported for pre-endoscopic RS were 3 [15], 4 [8, 14], and 5 [16], which also supports our cutoff of 4. One study [15] only reported deaths with scores higher than ours (pre-endoscopic RS ≥2 vs. 0, complete RS ≥4 vs. ≥1, and GBS ≥12 vs. ≥3), even though their mortality rate was similar to ours (6.7 vs. 5.9%).
Rebleeding is an important cause of longer length of hospital stay and readmissions. Regarding this outcome, results found in the literature are conflicting. Our study concluded that complete RS has the most discriminative value in predicting rebleeding. Both pre-endoscopic RS and GBS showed poor performance. Some articles described that none of these scores were helpful in predicting rebleeding [7, 8, 16]. Uysal et al. [15] report results similar to ours, where complete RS showed good performance in predicting this outcome, although the cutoff identified that divides patients into low and high risk of rebleeding groups was lower than the one we found: score of 5 versus 7. Chandnani et al. [17] concluded that GBS had better performance in this area than complete RS. This contradicts our findings, although it might be explained with their higher rebleeding rate (16.7 vs. 8.4%) [17].
The rate of transfusion calculated in this study was 51.7%. The European Society of Gastrointestinal Endoscopy (ESGE) [18] and the most recent recommendations from the International Consensus Group [19] recommend blood transfusion for target hemoglobin between 7 and 9 g/dL. A higher target should be considered in patients with significant comorbidities. However, we have not studied the relation between hemoglobin levels and transfusion, neither the number of units transfused. Our study concluded that GBS has the most discriminative value in predicting need for transfusion, while pre-endoscopic RS and complete RS showed insufficient performance. Mokhtare et al. [13] and Robertson et al. [16] describe similar results to ours. Various authors [14, 15, 17] concluded that pre-endoscopic RS and complete RS can also predict this need; however, they still found GBS to be a better discriminator. Regarding cutoff values to categorize patients into low and high risk of being transfused, there were conflicting results. Our study concluded that a score of 14 would be the best cutoff, although other studies found scores of 7-11 [13-16] to be more precise.
Regarding endoscopic intervention, there were fewer studies that included this endpoint. In our study, no score showed good performance in predicting this need, which was also concluded by Kim et al. [14]. However, some studies [8, 13] found GBS to have discriminative value in predicting this requirement.
The role of surgery in UGIB has been decreasing due to advances in endoscopy and endovascular therapies. As a result, surgical intervention is reserved when these have failed or when concurrent indications are present [20]. Our study concluded that none of the scores was useful in predicting the need for surgery. There were no other studies evaluating this specific need.
Regarding the need for hospitalization in IMCU or ICU, our study concluded that none of the scores had good performance in predicting this requirement. Some authors [14, 16] that only considered hospitalization in the ICU concluded that this need could be predicted with these scores, but all of them showed underwhelming results, with AUCs of 0.700 or 0.710. Mokhtare et al. [13] analyzed complete RS and GBS and described similar results to ours.
Appropriate health care is considered an intervention that is expected to do more good than harm to a patient with a health problem or set of problems, based on scientific evidence. The potential benefit or risk associated with any intervention varies according to the circumstances in which it is applied. In some cases, the risks and benefits of an intervention done in a particular patient will be easily predictable, but in others, there is a higher degree of uncertainty. Providing the best care to every patient is not always achievable for a variety of reasons, including availability of services, access to care, or variation between clinical practices. The purpose of this specialized endoscopy service is to pursue care that is safe, effective, efficient, patient-centered, timely, and equitable, allocating resources and clinical expertise in one acute care setting.
Overuse, underuse, misuse, and unjustified variation have been widely used to describe care that may be considered “inappropriate.” In this specific case, it is important to study the appropriateness of the transfers that are being made to this facility.
When evaluating the need for transfer, only pre-endoscopic scores can be used: pre-endoscopic RS or GBS. In our study, these scores were not significantly different in transferred patients when compared to directly admitted ones. However, the range of GBS is fairly different between the 2 groups (transferred patients had GBS ≥6 and directly admitted patients ≥0). This difference was not seen for pre-endoscopic RS. This might explain why most outcomes had higher rates in the transferred group, such as rebleeding, endoscopic intervention, surgery, and hospitalization in IMCU/ICU. On the other hand, mortality and transfusion rates were not significantly different between these groups.
Several articles [2, 12, 21] consider GBS to be the best score at determining very-low-risk patients, and they also concluded that patients who present scores ≤1 may be discharged. According to the ESGE, these do not require early endoscopy nor hospital admission [18]. Our study showed that there were no adverse outcomes when GBS was ≤3. With this information, when patients present these scores at admission, not transferring them can be carefully considered, at least not during the night, since transfers are not risk-free.
Our study only included transferred patients with GBS ≥6, but there were records of adverse outcomes in directly admitted patients with inferior scores. On the one hand, these results show that inter-hospital transportation, during the out-of-hours period, tends to be reserved for higher-risk patients with more probable need of endoscopic treatment. On the other hand, these findings might lead to the conclusion that there could have been patients that would have benefitted from being transferred to this facility but were not. Nevertheless, this conclusion needs to be considered with caution because this study did not include all the patients that were transferred. However, we can infer that all transfers were appropriate, and this issue reinforces the need for emergency endoscopy 24 h a day, 7 days a week.
This study has certain limitations, such as its retrospective nature and lack of some data, especially when patients were transferred to other facilities after endoscopy. Regarding blood transfusions, the number of units transfused and the relation between hemoglobin levels and transfusions were not studied.
Inconsistency in cutoff values amongst different studies is difficult to explain. However, this might be due to different characteristics in each study, including demographic data of patients, bleeding causes, time of endoscopy, and adherence to guidelines regarding endoscopic therapy. There are also differences between outcomes’ definitions compared to other studies, which makes comparison of results difficult.