Introduction
Adenomyomatosis (adenomyomatous hyperplasia, adenomyosis, or adenomyoma) is a rare benign lesion that has been observed in different sites throughout the gastrointestinal tract, most frequently in the gallbladder [1]. Adenomyomatosis of the gallbladder is most often an incidental finding during cholecystectomy performed for another reason with a prevalence of 1-9%, and large autopsy series report a prevalence of 7% [2,3]. Few cases have been described in the stomach, small bowel, bile ducts, and ampullary region. Adenomyomas of the vaterian system (ampulla of Vater [AV] and common bile duct [CBD]), unlike its counterparts in the rest of the digestive tract, have important clinical consequences, since the majority of these lesions present with biliary tract obstruction and mimic malignant behavior [1]. As consequence, despite being a benign lesion in most cases, patients are often treated with extensive surgery (pancreaticoduodenectomy). We report 2 cases of adenomyomatosis: one of the AV and the other of the CBD, as well as a review of cases reported in the literature. Both of our patients presented with epigastralgia and had laboratory or endoscopic evidence of biliary obstruction. The diagnosis of adenomyoma was only confirmed by the surgical specimen after cephalic pancreaticoduodenectomy.
Case Reports
Case 1
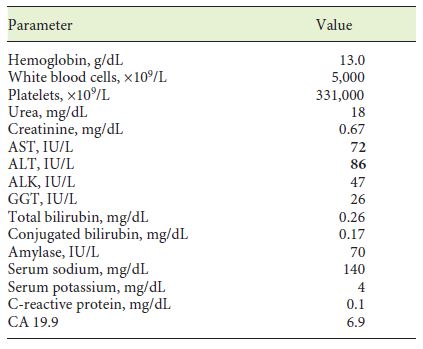
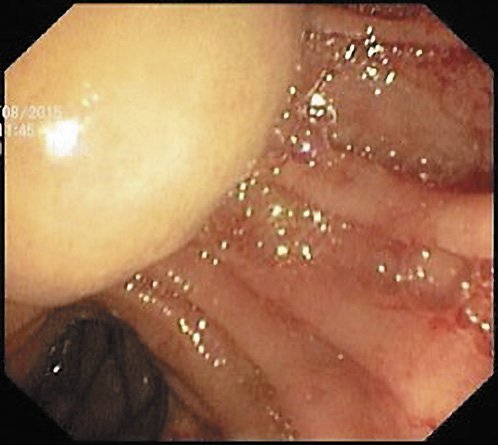
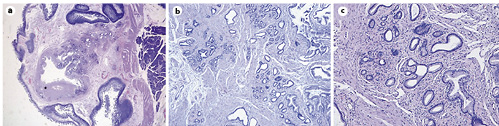
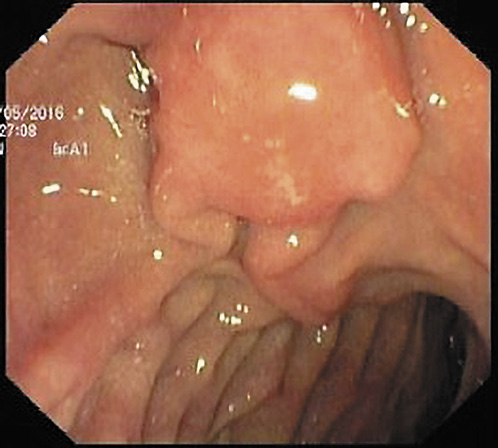
A 70-year-old woman with previous laparoscopic cholecystectomy for gallstones and a history of hypertension and dyslipidemia was referred to a gastroenterologist for epigastralgia and an abnormal abdominal CT scan, which revealed CBD dilatation (22 mm) with progressive reduction in size, without any AV or pancreas distortion. She had no family history of cancer and no jaundice. Laboratory workup showed elevated transaminases: 72 IU/L aspartate transaminase (AST) and 86 IU/L alanine transaminase (ALT). Alkaline phosphatase (ALK), γ-glutamyltransferase (GGT), total and conjugated bilirubin, and amylase were within normal ranges. Carcinogen antigen 19.9 (CA 19.9) was normal. She had a normal complete blood count and no elevation in acute-phase reactants (Table 1). A magnetic resonance cholangiopancreatography (MRCP) confirmed CBD dilatation with a localized stenosis 1 cm above the ampulla. A subsequently performed endoscopic ultrasound (EUS) showed a dilated CBD (16 mm) and a poorly defined hypoechogenic mass (1.5 × 1.9 cm) in the distal part. There was neither main pancreatic duct (MPD) or parenchyma involvement nor evidence of lymph node, ascites, or left hepatic lobe alterations (Fig. 1). A duodenoscopy showed a bulging AV with normal mucosa (Fig. 2). EUS-guided fine-needle aspiration (FNA) or brush cytology/biopsies obtained by endoscopic retrograde cholangiopancreatography (ERCP) was not performed because a negative or inconclusive histology would not change our therapeutic approach, since malignancy suspicion was high. The case was discussed at a digestive oncology multidisciplinary meeting and in consideration of the diagnostic hypothesis of cholangiocarcinoma of the distal bile duct and after discussion with the patient, she was submitted to a cephalic pancreaticoduodenectomy, which was performed 1 month later. Surgery was uneventful, and the patient was discharged on the 15th postoperative day. Macroscopic examination of the surgical specimen showed a bulging AV, CBP dilatation, and a subepithelial lesion without duodenal wall or pancreas invasion (Fig. 3). Histologically, the lesion consisted of hyperplastic glandular lobules surrounded by muscle fibers and fibroblasts, suggestive of adenomyomatosis of the CBP and AV (Fig. 4). At the 3-year follow-up, she was asymptomatic and without laboratory abnormalities.
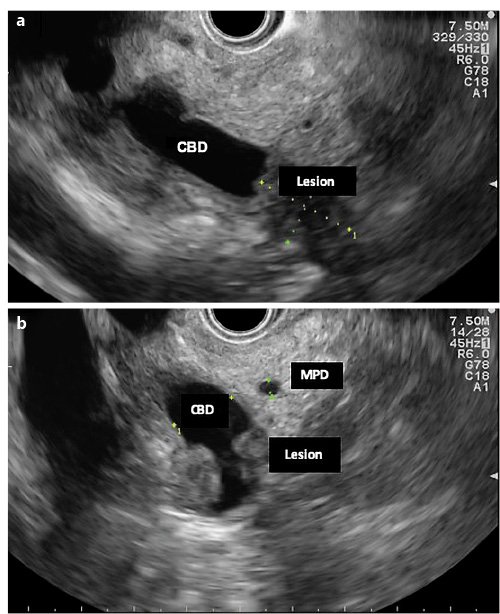
Fig. 1. EUS (linear endoscope) reveals dilated CBD and a poorly defined hypoechogenic mass in its distal portion (a).bMass in the distal common bile duct.
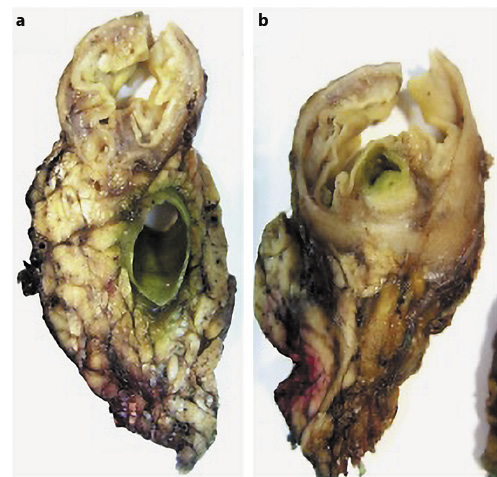
Fig. 3. Macroscopic examination of a surgical specimen shows bulging of the ampulla and CBD dilatation (a) and a subepithelial lesion without duodenal wall or pancreas invasion (b).
Case 2
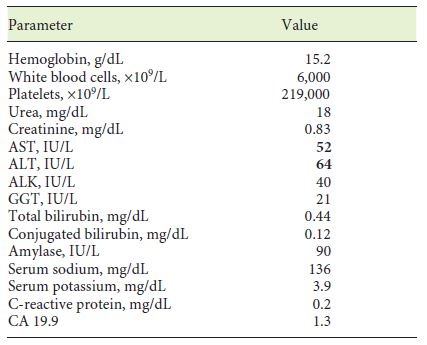
A 58-year-old man with a history of peptic ulcer disease and gastroesophageal reflux was referred to a gastroenterologist after an upper GI endoscopy, performed for epigastralgia. A protruding ampulla with a normal mucosa was described (Fig. 5). He had no family history of cancer and had no jaundice. Laboratory workup showed elevated transaminases with AST of 52 IU/L and ALT of 64 IU/L. ALK, GGT, total and conjugated bilirubin, and amylase were within normal ranges. A complete blood count was normal, and acute-phase reactants were not elevated. CA 19.9 was normal (Table 2). EUS showed a 12-mm, poorly defined, hypoechogenic mass in the AV area, with involvement of the distal CBD and the muscular layer of the duodenal wall. There was no evidence of CBD dilatation or pancreatic involvement (Fig. 6). FNA was performed. Cytological examination showed groups of epithelial cells: some with benign characteristics and others with nuclear overlap and increased nuclei, in favor of epithelial dysplasia, without obvious carcinoma characteristics (Fig. 7). The case was discussed at a digestive oncology multidisciplinary meeting; considering the diagnostic hypothesis of ampulloma and after discussing the case with the patient, she was submitted to cephalic pancreaticoduodenectomy, which was performed 1 month later. The surgery was uneventful, and the patient was discharged on the 8th postoperative day. Macroscopic examination of the surgical specimen showed a white and firm tumor of 1.6 cm in largest diameter (Fig. 8). Histologically (Fig. 9), there was a slight CBD and MPD dilatation and some inflammatory infiltrate. The ampulla consisted of aggregates of ductal proliferation surrounded by fibrosis, which had continuity with the muscular layer of the duodenal wall. There were some areas with enlarged and stratified nuclei in favor of reactive atypia. These findings were consistent with the diagnosis of AV adenomyomatosis. At the 2-year follow-up, she was asymptomatic and without analytical alterations.
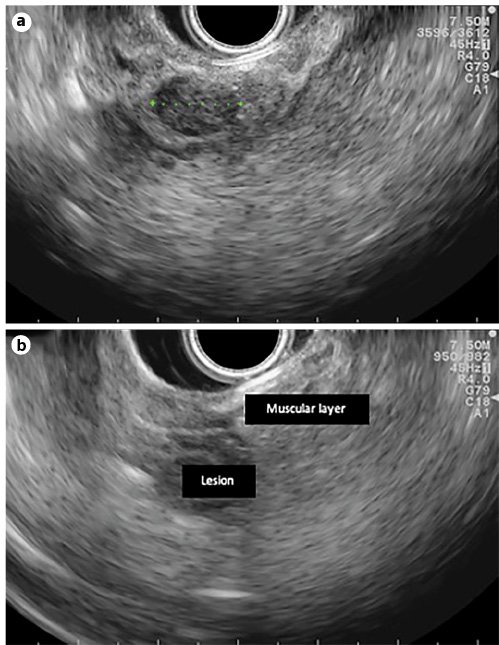
Fig. 6. EUS features (linear endoscope): 12-mm hypoechogenic mass in the ampulla area (a) and a lesion with duodenal-wall muscular-layer involvement (b).
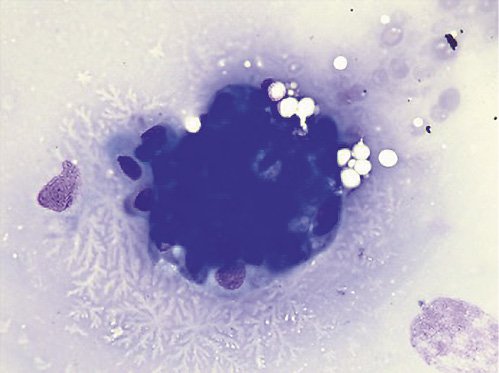
Fig. 7. Cytology examination showed groups of epithelial cells: some with benign characteristics and others with nuclear overlap and increased nuclei in favor of epithelial dysplasia without obvious carcinoma characteristics.
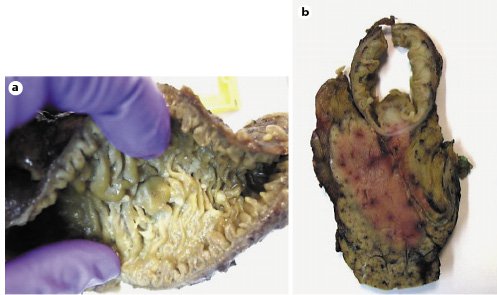
Fig. 8. Macroscopic examination of a surgical specimen: bulging ampulla (a) and white and firm tumor 1.6 cm in the largest diameter (b).
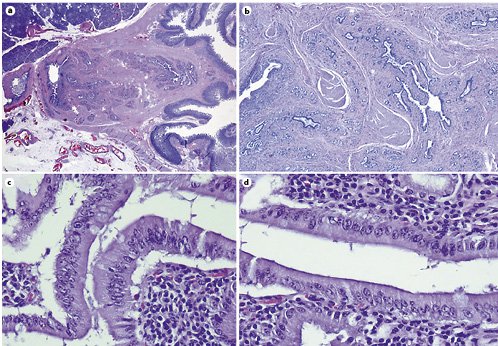
Fig. 9. Microscopic examination of the specimen. H&E.aLow magnification showing that the lesion consisted of aggregates of ductal proliferation surrounded by fibrosis and had continuity with the muscular layer of the duodenal wall.bLow magnification demonstrates slight dilatation of CBD and MPD, and chronic periductal inflammatory infiltrate.c,dAreas with enlarged and stratified nuclei in favor of reactive changes. ×40.
Discussion
According to the WHO classification, adenomyoma is a benign lesion with no premalignant risk, defined as duct-like structures accompanied by hyperplasia of smooth muscle bundles [4]. The real incidence of these lesions is difficult to settle as different names are used to designate the same histological lesion [1]. Published series of unselected postmortem examinations report an incidence of 50-70% of small adenomyomas of the vaterian system (<5 mm), a high percentage of cases having no relevant associated clinical history. Symptomatic lesions reported in the medical literature are much rarer and reported mostly as single case reports [5,6]. To our knowledge, this is the first literature review regarding AV and CBD adenomyomatosis. Our 2 cases were a 70-year-old woman and 58-year-old man with laboratory or endoscopic evidence of biliary obstruction, in whom preoperative diagnosis was ambiguous, and the diagnosis of adenomyoma was only confirmed by the surgical specimen after pancreaticoduodenectomy.
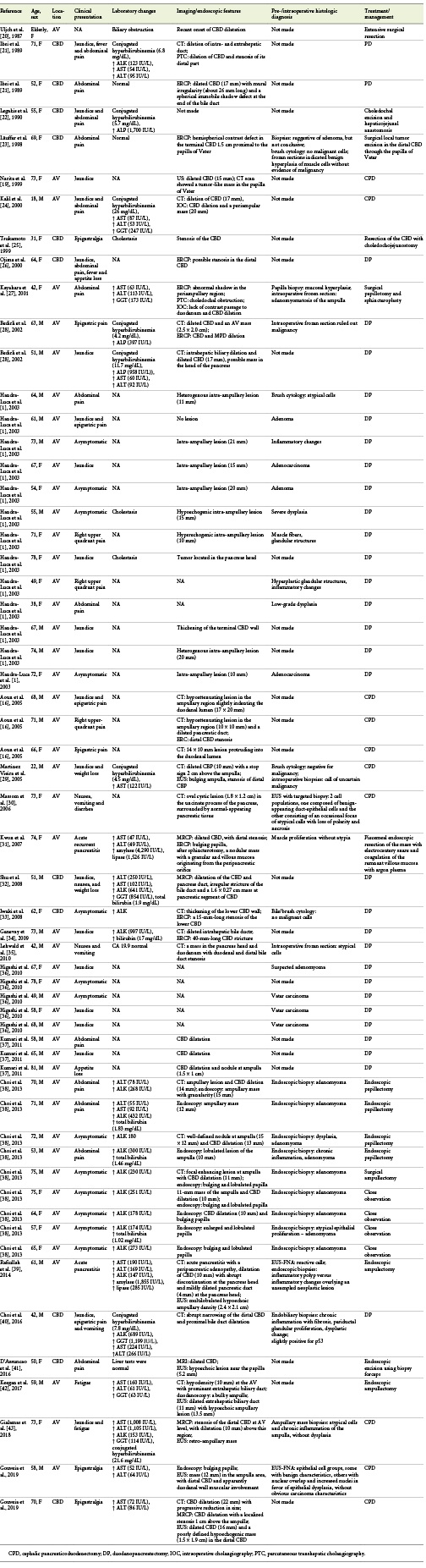
A PubMed search conducted using the key words “adenomyomatous hyperplasia,” “adenomyoma,” “adenomyosis,” “adenomyomatosis,” and “ampulla of Vater” or “common bile duct,” revealed 61 case reports (from 1987 to July 2018) eligible for analysis (Table 3). Regarding published cases, almost half of the patients were male (n= 29, 48%), and their mean age was 62 years (range 18-81 years). Forty-nine patients had AV adenomyoma (80%), and 12 had CBD adenomyoma (20%). Patients presented with jaundice (n= 22/61), abdominal pain (n= 25/61), nausea and vomiting (n= 3/61), acute pancreatitis (n= 2/61) - both with AV lesions, loss of appetite (n= 3/61), and fatigue (n= 1). Fifteen patients (25%) were asymptomatic, and the finding was incidental. For 1 patient, clinical presentation was not mentioned. Nineteen patients had cholestasis/conjugated hyperbilirubinemia, 10 had transaminase, ALK, or GGT elevation with normal bilirubin, 2 patients had elevated amylase and lipase, and 3 patients had normal liver tests. In 26 cases, laboratory workup was not reported. Imaging (abdominal CT, MRI, and MRCP) and endoscopic (ERCP and upper GI EUS) features more frequently found were CBD or MPD dilation, tumor-like mass in the papilla region or distal CBD, CBD stenosis, intrahepatic biliary tract dilation, and bulging papilla (in patients with ampullary lesions). There was no preoperative or intraoperative histological diagnosis in 26 patients. In the other patients, several different diagnoses were made: 2 adenomas, 5 adenocarcinomas, 2 cases of inflammatory changes, 3 cases of dysplasia, 3 cases of atypical cells, 3 cases of muscular and glandular proliferation, 1 case of suspected adenomyoma, 8 adenomyomas, 1 adenomyoma with dysplasia, and 1 patient without malignant cells. Besides these pre-/intraoperative diagnoses, in 6 patients, intraoperative frozen sections revealed adenomyomatosis of ampullary, glandular, and muscular proliferation, muscle-cell hyperplasia, uncertain for malignancy, atypical cells, and negative for malignancy, respectively. Consequently, only in 9 patients (15%), adenomyoma was diagnosed pre-/intraoperatively. These patients were submitted to endoscopic papillectomy (n= 4), surgical papillectomy (n= 1), and close observation (n= 4). Forty-one patients (67%) underwent duodenopancreatectomy, 7 patients were submitted to endoscopic ampullectomy, 2 patients underwent surgical ampullectomy, 2 had local surgical/extensive excision, 2 had CBD surgical resection, 1 had endoscopic mass excision using biopsy forceps, and 4 patients received close observation with repeated endoscopic observations (lesions did not change with time, but the duration of follow-up time is mentioned in the case report).
Table 3 Literature review of adenomyomatosis of the ampulla of Vater (AV) and common bile duct (CBD)
The diagnosis of adenomyoma of the vaterian system (AV and CBD) is challenging. Patients often present with signs of biliary obstruction and cholestasis, and preoperative imaging (CT, MRI, and MRCP) frequently shows common bile duct obstruction or a tumor-like mass. Endoscopic biopsies, EUS-FNA and brush cytology show most of the time atypical cells, dysplasia, or even malignancy. In retrospect, these findings are thought to be secondary to AV and CBD endoscopic manipulation (biopsy, brush cytology, and sphincterotomy), and may contribute to the diagnostic difficulties. The overall accuracy for preoperative histopathological diagnosis with endoscopic forceps biopsies in patients with AV tumors was reported as 62% by Menzel et al. [7]. Hammarström et al. [8], in a study including 3,131 patients submitted to ERCP, showed that a correct endoscopic diagnosis was only made in 2 of the 4 patients with adenomyoma. ERCP also allows for brush cytology and intraductal biopsy performance. The sensitivity of brush cytology and intraductal biopsy in diagnosing malignant biliary strictures are reported as 45 and 48.1% respectively, and both techniques are almost 100% specific. A combination of both modalities modestly increased the sensitivity to 59.4% [9]. To overcome this limitations, Kim et al. [10] and Uchida et al. [11] showed that repeated testing (multiple cytology tests) via endoscopic nasobiliary drainage increased the cumulative diagnostic rate, with a sensitivity of 95% with 6 repeated exams [10,11]. Logrono et al. [12], who analyzed 183 pancreatobiliary brush specimens from 2 university hospitals, showed that the possibility of malignancy with no evidence of malignancy from repetitive endoscopic biopsy was lower than 10%. EUS-FNA can be performed for distal extrahepatic bile duct strictures, with a sensitivity and negative likelihood ratio for diagnosis of malignancy of 66% and 0.34, respectively [13]. Furthermore, EUS-FNA can be performed in ampullary and distal CBD masses with an overall accuracy of 100%, with a sensitivity, specificity, and positive and negative predictive values of 100% [14]. Intraoperative frozen sections from the mass can usually differentiate whether the lesion is benign or malignant (adenomyoma and adenocarcinoma). However, most pathologists have limited experience with frozen-section adenomyomas [15]. Macroscopically, adenomyoma of the ampullary region usually appears as a rounded, well-defined, intraluminal lesion arising from the CBD wall, although some case reports have described a diffuse form infiltrating the CBD wall which resembles a stenotic lesion [16]. The histological aspect of adenomyoma is characterized by multiple lobules of glands, mainly located in the muscle layers of the vaterian system. The lobular formations consist of small glands arranged around a larger gland and surrounded by myofibroblastic and fibroblastic proliferation. This mesenchymal component is rather composed of fibroblasts and myofibroblasts (with smooth muscle actin expression but without desmin expression), but it may contain sparse smooth muscle cells [1]. The histogenesis of adenomyoma and adenomyomatous hyperplasia is still a subject of controversy. The most widely accepted hypothesis is that these lesions may represent a form of incomplete heterotopic pancreas (type III), as described by von Heinrich in 1909 [1]. The presence of hyperplastic smooth muscle tissue can be explained by secondary muscle proliferation caused by some stimulus emanating from misplaced epithelium, by muscle misarrangement, or by aberrant growth invading and distorting normal muscle [1]. Martin et al. [17] compared adenomyoma of the vaterian system to its gallbladder counterpart and claimed that the former is a lesion developed in diverticula, accompanied by reactive muscle hyperplasia and secondary gland formation, which leads to poorly defined lobules. Fernandez-Cruz and Pera [18] considered adenomyoma as part of an involutive process of fibroadenomatous type due to increasing age. Other authors, such as Narita and Yokoyama [19], stress the possibly inflammatory nature of this lesion.
Conclusion
Adenomyomatosis of CBD and AV are rare benign lesions, which pose a diagnostic challenge, as they often present with biliary obstruction and mimic malignant neoplasms; imaging and endoscopy rarely offer a definitive diagnosis. As a consequence, in most cases, patients are treated with extensive surgery despite its benign nature. The development and application of new endoscopic, radiological, and pathological modalities are necessary in order to improve the diagnosis and management of these lesions.