Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
GE-Portuguese Journal of Gastroenterology
Print version ISSN 2341-4545
GE Port J Gastroenterol vol.27 no.6 Lisboa Dec. 2020
https://doi.org/10.1159/000507203
IMAGES IN GASTROENTEROLOGY AND HEPATOLOGY
A Pancreatic Cyst Leading to Obstructive Jaundice
Icterícia obstrutiva associada a quisto pancreático
Catarina Sousa Félixa, Susana Chaves Marquesb, Miguel Bispob, Mireia Castilloc, Tiago Bana e Costaa, Cristina Chagasa
aGastroenterology Department, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; bGastroenterology Department, Champalimaud Foundation, Lisbon, Portugal; cPathology Department, Champalimaud Foundation, Lisbon, Portugal
* Corresponding author.
Keywords: Pancreas, Pancreatic cyst, Pancreatic neoplasm, Endoscopic ultrasound-guided fine-needle aspiration
Palavras-Chave: Pâncreas, Quisto pancreático, Neoplasia pancreática, Aspiração por agulha fina guiada por ultrassonografia endoscópica
71-year-old female patient presented with new-onset jaundice (total bilirubin 7.39 mg/dL, conjugated bilirubin 5.38 mg/dL), choluria, and acholia. Her past medical history was remarkable for a pancreatic head cystic lesion diagnosed in 2009; it was a 24-mm multilocular cystic lesion, with no communication with the pancreatic duct or dilatation of this duct, with a central scar that remained asymptomatic and stable in size until 2015, when follow-up was lost. An abdominal ultrasound revealed a 47 × 45 mm heterogeneous mass in the pancreatic head, associated with de novo common bile duct (CBD) dilatation (17 mm in the liver hilum).
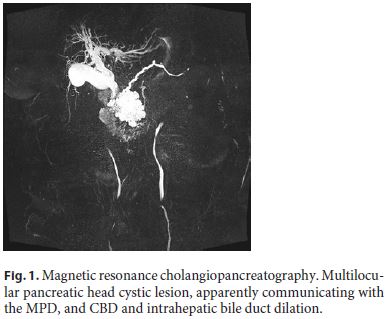
Magnetic resonance cholangiopancreatography showed a multilocular pancreatic head cystic lesion, measuring 60 × 70 × 57 mm and apparently communicating with the main pancreatic duct (MPD), without evident central scar. There was also CBD and intrahepatic bile duct dilation, as well as pancreatic body and tail atrophy (Fig. 1).
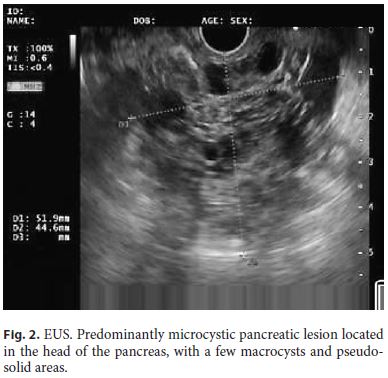
Endoscopic ultrasound (EUS) documented a predominantly microcystic lesion located in the head of the pancreas, measuring 53 × 46 mm, with a few macrocysts and pseudo-solid areas, compressing the CBD and the superior mesenteric vein, and without vascular invasion or Wirsung dilatation (Fig. 2). Fine-needle aspiration of the macrocyst and of a pseudo-solid area was performed. Cyst fluid biochemistry analysis revealed a high amylase level (54,085 UI/L) and a low CEA (2 ng/mL). EUS-fine-needle aspiration cytology showed cuboidal cells, negative for CK8/18, synaptophysin, and chromogranin-A (Fig. 3). For jaundice palliation, endoscopic retrograde cholangiopancreatography (ERCP) was then performed, and a plastic stent was placed. During ERCP, incidental pancreatic duct cannulation and contrast injection occurred, and pancreatography showed a multicystic lesion in the head of the pancreas and confirmed MPD communication (Fig. 4).
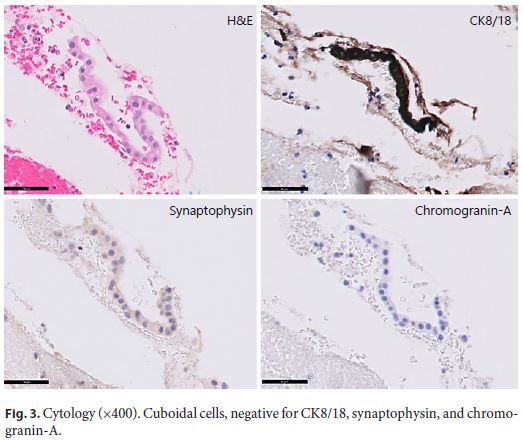
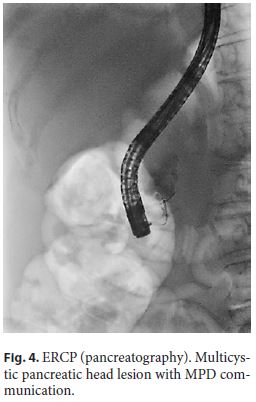
At this point, differential diagnosis include sidebranch intraductal papillary mucinous neoplasm, mucinous cystic neoplasm, serous cystic neoplasm and pseudopapillary neoplasm. The previously reported scar, the low CEA, and the presence of cuboid epithelium pointed to a serous cystic neoplasm. Despite the uncertain diagnosis, the presence of high-risk stigmata lead to surgical resection (Whipple procedure), confirming the diagnosis of a serous cystic neoplasm. After the surgery, complete resolution of the symptomatology was noted.
Serous cystic neoplasms account for 8–17% of the clinically encountered pancreatic cystic lesions and are predominantly found in middle-aged females [1]. These lesions are usually benign and asymptomatic and present a benign clinical course in the vast majority of patients [2]. Symptoms may occur, typically in those patients with lesions > 4 cm in size, and are often attributed to mass effect or to infiltration of adjacent structures. Abdominal pain (25%), palpable mass (10%), and jaundice (7%) are the main clinical manifestations. In such cases, symptomatic surgical resection should be considered, and once resected, no further surveillance imaging is needed [3].
In the reported case, presurgical diagnosis was not straightforward, mainly due to the presence of pseudosolid areas and cystic communication with the MPD with subsequent high amylase, which is a rare feature of serous cystic neoplasm. Although rare, communication with the MPD has been reported occasionally (0–0.6%) in serous cystic neoplasm, requiring a strong suspicion, so that the misdiagnosis of an intraductal papillary mucinous neoplasm is avoided [1].
References
1 Matsubayashi H, Oka Y, Ito T, Uesaka K, Sasaki K, Ono H. A Case of Serous Cystadenoma Communicating with a Stenotic Santorini’s Duct and a Dilated Main Pancreatic Duct. J Gastrointestin Liver Dis. 2016 Dec;25(4):551–4.
2 Amico EC, Alves JR, de Araújo Lima Liguori A, Sousa RL. Serous Pancreatic Cystadenoma with Compression of Wirsung’s Duct. J Gastrointest Surg. 2019 Jan;23(1):176–8.
3 Zhang XP, Yu ZX, Zhao YP, Dai MH. Current perspectives on pancreatic serous cystic neoplasms: Diagnosis, management and beyond. World J Gastrointest Surg. 2016 Mar;8(3):202–11.
Statement of Ethics
Informed consent was obtained from the patient for the case publication.
Disclosure Statement
The authors have no conflicts of interest to declare.
Funding Sources
The authors have no funding sources to declare.
* Corresponding author.
Catarina Sousa Félix
Gastroenterology Department, Centro Hospitalar Lisboa Ocidental
Rua da Junqueira 126
PT–1349-019 Lisbon (Portugal)
Received: November 10, 2019; Accepted: January 20, 2020
Author Contributions
Catarina Sousa Félix collected the data and wrote the manuscript; Susana Chaves Marques, Miguel Bispo, Mireia Castillo, Tiago Bana e Costa, and Cristina Chagas revised the manuscript and approved the final version.














