Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
GE-Portuguese Journal of Gastroenterology
versão impressa ISSN 2341-4545
GE Port J Gastroenterol vol.27 no.5 Lisboa out. 2020
https://doi.org/10.1159/000505035
RESEARCH ARTICLE
Predictive Factors and Clinical Impact of Deep Remission in Celiac Disease
Fatores preditivos e impacto clínico da remissão profunda na doença celíaca
Marta Silvaa, Armando Peixotoa–c, Ana Luísa Santosa–c, Pedro Costa-Moreiraa–c, Joel Ferreira da Silvaa–c, Emanuel Diasa–c, Guilherme Macedoa–c
aPorto Medical School, University of Porto, Porto, Portugal; bGastroenterology Department, Centro Hospitalar de São João, Porto, Portugal; cPorto WGO Training Center, Porto, Portugal
* Corresponding author.
ABSTRACT
Introduction: The ultimate indicator of adherence to a gluten-free diet is the demonstration of mucosal healing. However, the need for histological reassessment is subject to controversy among “experts”. The aim of this study was to evaluate celiac patients who underwent histological reevaluation after starting a gluten-free diet in order to identify those with histological remission and associated factors. Methods: This retrospective study included patients who agreed to a histological reassessment after apparent clinical and serological remission and reported at least 12 months of diet adherence. In all cases, informed consent was signed for upper endoscopy. Results: A total of 69 patients were included. In 67.9% of cases, the diagnosis was made in the context of “classic” symptomatology, 17% had “nonclassical” presentation, and 15.1% were in latent phase. 69.2% of the diagnoses were initially suspected by serology. Endoscopically, 11.8% of the patients did not present suggestive features macroscopically, and a histological grade of Marsh IIIa–c was observed in 75.5% of all cases. The histological findings were normalized in 37.7%, which was associated with the presence of lower Marsh score values at diagnosis (p = 0.014) and lower DEXA T-score values (p = 0.038). A histological improvement was observed in 55 patients (≥2 grades in 37 cases), which was related to the initial transferrin saturation (p = 0.027) and with higher Marsh scores at diagnosis (p = 0.007). Conclusion: Even under a gluten-free diet, celiac histology normalization is difficult to obtain and appears to be independent of most clinical and serological findings at diagnosis. Patients with less severe histological levels at diagnosis reach remission more easily, but only represent the minority of the population.
Keywords: Celiac disease, Deep remission, Endoscopic reassessment, Gluten-free diet
RESUMO
Introdução: O indicador final da adesão a uma dieta isenta de glúten é a demonstração da cicatrização da mucosa. No entanto, a necessidade de reavaliação histológica é um assunto controverso entre “experts.” O objetivo deste estudo foi avaliar doentes celíacos submetidos a reavaliação histológica após o início da dieta isenta de glúten, a fim de identificar aqueles com remissão histológica e fatores associados. Métodos: Estudo retrospectivo, incluindo doentes que concordaram com reavaliação histológica após aparente remissão clínica e serológica, com pelo menos doze meses de adesão relatada à dieta. Em todos os casos, o consentimento informado foi assinado para endoscopia digestiva alta. Resultados: Um total de 69 doentes foram incluídos. Em 67.9% dos casos, o diagnóstico foi feito no contexto de sintomatologia clássica, 17% de apresentações não clássicas e 15.1% em fase latente. A maioria (69.2%) dos diagnósticos foram inicialmente suspeitos com base na serologia. Na endoscopia, 11.8% dos pacientes não apresentavam características macroscópicas sugestivas de doença celíaca, observando- se um grau histológico de Marsh IIIa-c em 75.5% dos casos. Os achados histológicos normalizaram em 37.7% dos doentes, o que foi associado à presença de menores valores de Marsh no momento do diagnóstico (p = 0.014) e menores valores no T-score da densitometria óssea (p = 0.038). Melhoria histológica foi observada em 55 doentes, em dois ou mais graus em 37 casos, o que se relacionou com a saturação inicial da transferrina (p = 0.027), e com maiores scores de Marsh no momento do diagnóstico (p = 0.007). Conclusão: Mesmo sob uma dieta isenta de glúten, a normalização da histologia na doença celíaca é difícil de obter e parece ser independente da maioria dos achados clínicos e serológicos no momento do diagnóstico. Doentes com níveis histológicos menos graves ao diagnóstico alcançam a remissão mais facilmente, mas representam apenas a minoria da população.
Palavras-Chave: Doença celíaca, Remissão profunda, Reavaliação endoscópica, Dieta isenta de glúten
Introduction
Celiac disease (CD) is a chronic autoimmune disease that is triggered by gluten intake in genetically predisposed individuals [1]. CD can affect all age groups [2], with an overall prevalence of 1% [3, 4]. As there is growing knowledge and awareness about CD and its manifestations, there has been an increase in its incidence, particularly in Western [5, 6]. Although it is a multisystemic disease, it mainly affects the small intestine [7], presenting a wide clinical spectrum, which may include typical/classic manifestations secondary to intestinal malabsorption and atypical/nonclassical manifestations, where the extraintestinal symptoms are included, as well as the absence of symptoms (subclinical disease) [8].
Currently, the only established treatment for these patients is to maintain a gluten-free diet (GFD) throughout their lives [9]. Indeed, when gluten is ingested, celiac patients suffer an increase in serum levels of certain antibodies, such as IgA antitransglutaminase, which is the most commonly used marker for diagnosis because it has a high sensitivity and specificity [10]. In addition, as these antibodies tend to normalize with strict GFD maintenance, they become useful in monitoring patients after the onset of the diet and over time. However, although decreasing concentrations of CD-specific antibodies indicate a reduction in gluten intake, they have a limited ability to define complete compliance, and there is evidence that small amounts of gluten exposure may not be detected in this way [11]. Once antibody titers have normalized, a subsequent increase in their levels is considered a good indicator of undue gluten intake, which is currently widespread [12].
Despite the usefulness of serology in the follow-up of CD patients, the ultimate indicator of adherence to the diet is the demonstration of mucosal healing, although this may not occur even in compliant patients. The need for duodenal biopsies to assess healing and GFD adherence is a subject of controversy among experts [13–16]. Although this approach is often used in clinical practice, it is not clear whether it is necessary in patients who are clinically responsive and who show decreasing or negative levels of autoantibodies [1, 6, 17]. Among those recommending repeated biopsy, the timing for when samples should be obtained is not well defined [18]. Complete healing of the intestinal mucosa is also often slow or incomplete, especially among adults [19]. The maintenance of villous atrophy can lead to persistent nutritional deficiencies or complications such as osteoporosis, or it can mimic the irritable bowel syndrome [11]. Recently, it has been shown that the majority of CD patients do not have a satisfactory histological response [19], and, therefore, duodenal biopsy may be the only tool capable of identifying these patients and consequently those with a potential risk of complications. Current evidence on this issue is scarce, and further studies are required to determine whether histological reassessment should be on a routine basis, and, in whom confirmation of mucosal healing may be important in clinical decision making.
This research project aimed to epidemiologically characterize the population of CD patients followed in a specialist consultation in the Department of Gastroenterology of the Sao Joao Hospital Center. In particular, it was intended to study the patients submitted to histological reevaluation after initiation of GFD in order to identify those that present histological improvement, including complete remission of the mucosa and demographic, clinical, and therapeutic factors associated with these outcomes.
Materials and Methods
Study Design
After ethics committee approval, a retrospective study was carried out without any intervention in the population. Indeed, in this investigation, endoscopic reassessment was proposed by the attending physician of the CD patients under study after explaining that their achievement is still controversial and as such optional.
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a prior approval by the institution’s human research committee. Written, informed consent was obtained from each patient included in the study.
Participants
Thus, the study sample included all patients diagnosed with CD (based on the criteria of the World Gastroenterology Organization Global Guidelines, 2016 [1]) followed by a specialized consultation in the Gastroenterology Department of the Sao Joao Hospital Center. In total, 161 patients with a confirmed diagnosis of CD were studied. In this study, we specifically assessed patients in apparent clinical remission (indicated by a normalization of the antibody titers after at least 12 months of reported GFD) who, at follow-up, agreed to undergo histological reassessment through endoscopy with duodenal biopsies. The study included female and male patients aged 18 years or older. The follow-up of celiac patients in the consultation follows the algorithm proposed by the American College of Gastroenterology and published in their guidelines in 2013 [20].
Data Extraction
The data needed to carry out this research were obtained from the electronic clinical records of the patients, including clinical diaries and laboratory and imaging examinations performed by patients.
Statistical Analysis
The clinical data obtained in this project were stored in a database using SPSS, which was later subjected to treatment and statistical analysis in order to evaluate the proposed objectives. Subsequently, a descriptive analysis of the same data was carried out in order to answer the research questions. Categorical variables were characterized by relative frequency and studied using the Pearson χ2 test. Since there was no normal distribution between groups, continuous variables were studied using the Mann-Whitney U test. For groups of variables with a small number, Fisher’s exact test was used. For all comparisons, a value of α of less than 0.05 was considered statistically significant.
Study Variables
Based on the July 2016 WGO guidelines [1], patients were classified as classical, nonclassical, and subclinical CD patients, depending on the symptoms they showed at the time of diagnosis. Symptoms of classical disease included chronic diarrhea, weight loss, iron deficiency anemia, abdominal distension, malaise, and fatigue, edema, osteoporosis, growth retardation, vomiting, sarcopenia, and irritability. The symptoms of nonclassical disease included abdominal pain, CD, chronic fatigue, constipation, chronic migraine, dermatological manifestations, peripheral neuropathy, hypertransaminasemia, folic acid deficiency, bone density reduction (by DEXA scan at diagnosis), infertility, pubertal delay, late menarche and early menopause, dental alterations, dyspepsia, and psychopathy.
Regarding the diagnosis, this depends on the combination of several factors, namely the clinical history, physical examination, presence of specific antibodies, and compatible intestinal biopsy. Thus, IgA antitissue transglutaminase antibody was used in serology, the value of which was considered negative when < 7.0 U/mL. In IgA-deficient patients, an IgG assay was performed. Regarding the histological evaluation, the included endoscopic reassessment biopsies were performed 12–24 months after the initiation of GFD, and the Marsh classification was used to classify them. All patients with Marsh I and II had serology compatible with CD.
Deep remission was defined by the absence of histological findings suggestive of the disease, described as Marsh 0. Histological improvement was defined by reducing at least one value in the Marsh score, including those in histological remission.
Results
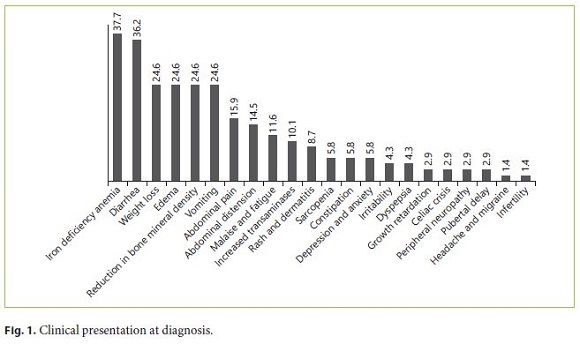
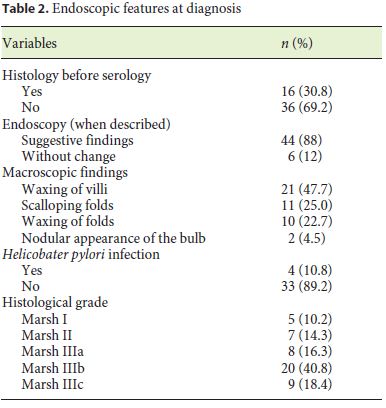
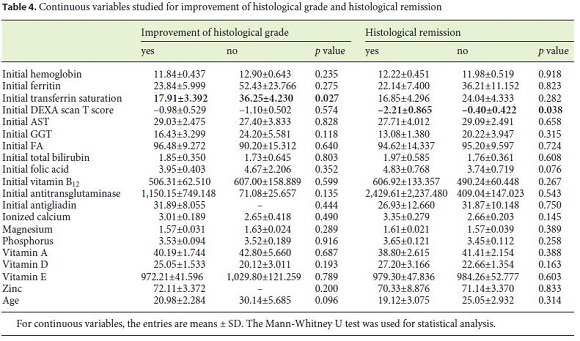
During the investigation, 69 patients met the inclusion criteria. Most of the CD patients were female (79.7%). The mean age at the time of diagnosis was 22.5 years. All patients were Caucasians. In terms of age distribution at diagnosis, 36.2% of the cases were diagnosed at pediatric age. The most frequent age group was between 18 and 39 years (42%), followed by those 40–65 years old (20.3%). A minority was diagnosed between 11 and 17 years (5.8%), and only 1 case (1.4%) was older than 65 years. For more detailed information regarding the age groups consult Table 1. Based on the July 2016 WGO guidelines [1], 36 (67.9%) were classified as classical, 9 (17.0%) nonclassical, and 8 (15.1%) in latent phase. Regarding family history, 15.9% of the patients had celiac relatives, 9 had first-degree relatives, and 1 had second-degree relatives. A minority of patients (7.2%) had concomitant diagnosis of other autoimmune diseases: 2 cases of diabetes mellitus and 3 cases of autoimmune thyroiditis. There were no autoimmune hepatitis or gastritis, Down, Turner, or Williams syndrome observed in the study population. In terms of complications, only 5.8% were affected, 1 of the patients had type II refractory CD, another had ulcerative jejunitis, and the other 2 osteoporosis. Most of the patients (76.8%) had a total diet compliance. 29.9% had nutritional deficiencies at the time of diagnosis (Table 1). The frequencies of the clinical presentations studied can be analyzed in Figure 1. It is important to highlight iron deficiency anemia (37.7%) and diarrhea (36.2%), which were the most commonly reported symptoms during the investigation (Fig. 1). Only 30.8% of the population performed endoscopy before serology, and in most patients the diagnosis was initially suspected by serology. At endoscopy, 11.8% of the patients did not present macroscopic features suggestive of CD, and only 10.8% had Helicobacter pylori infection. A Marsh IIIa–c histological grade was observed in 75.5% of all cases (Table 2).
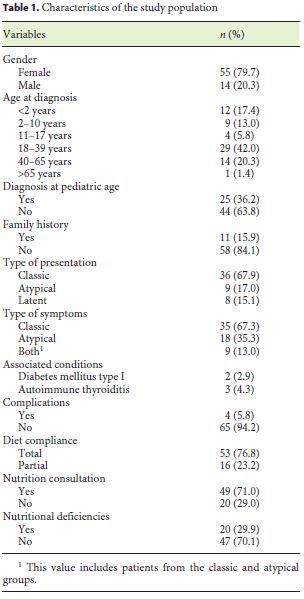
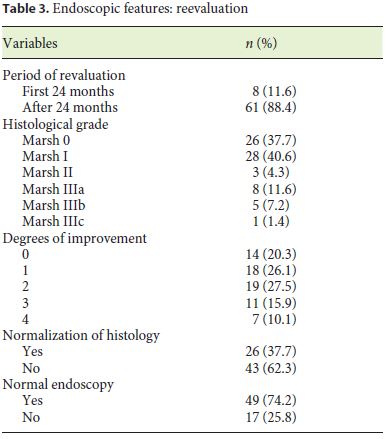
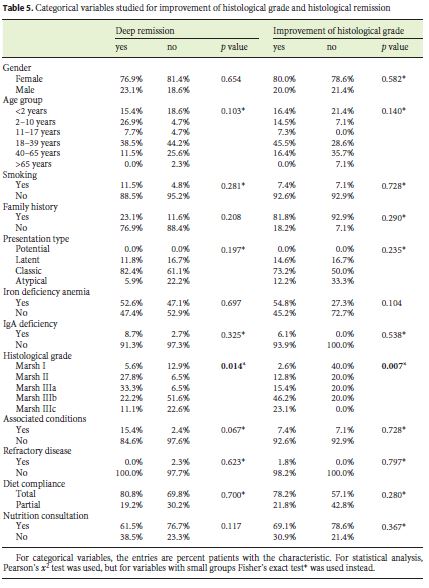
Eight patients (11.6%) underwent reassessment endoscopy within 24 months of starting the diet, and the remainder after 24 months. The average time between the start of GFD and reassessment endoscopy was 7.98 (±5.6) years. The histological findings were normalized in 37.7% (n = 26), which was associated with the presence of lower Marsh score values at diagnosis (p = 0.014) and a statistical trend for the presence of other autoimmune conditions (p = 0.067). Deep remission was also associated with lower DEXA T-score values (p = 0.038). A histological improvement over baseline was observed in 55 patients (79.6%), of 2 or more grades in 37 cases, which was related to a lower initial transferrin saturation (p = 0.027) and with higher values of the Marsh score at diagnosis (p = 0.007) (Tables 3, 4, 5).
Discussion
Duodenal histological revaluation after GFD adherence is still a controversial subject [13–16] but one with a major significance in the field of CD investigation. In fact, there are some experts who argue that profound remission can take a long time or even not happen [19]. In this investigation, we tried to determine the clinical factors related with histological improvement and deep remission. This investigation may indicate if histological reassessment should be routinely performed or not, and who could clinically benefit from this revaluation.
In this sense, during this study, we analyzed the correlations between multiple variables related to CD and histological improvement or deep remission. In fact, the study showed that there is a statistically significant relationship between histological improvement and lower transferrin saturation levels at the time of diagnosis (17.91 vs. 36.25%, p = 0.027). This may be explained by the fact that patients with lower levels of transferrin saturation may represent a more severe spectrum of the disease and therefore be more susceptible to improvement after starting the diet.
We also showed a statistically significant relationship between the histological grade at the reassessment endoscopy and the T score in bone densitometry (p = 0.038). In fact, patients with deep histological remission had a mean T score of –2.21 ± 0.865, and those who did not show deep remission had a mean T score of –0.40 ± 0.422. This is na unexpected finding given that one of the frequent signs of CD is the premature reduction in bone density [21]. Such a result may be related to the fact that celiac patients without improvement have closer surveillance of their bone density and begin treatment earlier. In addition, bone density is dependent on several other factors, including sex, age, smoking, medication, and genetics, factors for which this association was not controlled.
Evidence also indicated that there was a trend towards a relationship between histological normalization and CDassociated autoimmune conditions (p = 0.067). In this case, there may have been a significant loss of statistical power because of the limited number of patients in the study.
On the other hand, it was found that the presence of lower Marsh score values at diagnosis was significantly associated with histological normalization (p = 0.014). Probably, this may be due to the fact that the higher the histological grade, the more difficult it is to obtain a complete recovery of the duodenal mucosa. This is in accordance with what is now known, which makes it difficult to find a justification for the need to prove deep remission in all patients within a certain time period. In fact, it is not known how much time each patient needs for histological remission [18, 22].
Finally, it was found that only a third of the patients reached deep histological remission, regardless of clinical and serologic response, which indicates that, as Wahab et al. [19] argues, deep remission may take a long time, especially in adulthood, or it may not even be reached by some patients, without relevant clinical impact. In addition to the absence of histological remission in most patients, no clinical factors were found to be related to the absence of this remission. In fact, during this investigation, none of the clinical variables, including complications, were related to outcome. This further complicates the identification of the patients who benefit from the histological reevaluation, stressing the controversy of this issue. As such, the challenge will be to select the patients for whom that evidence may be beneficial for follow-up. Given that, this may lead us to rethink a more appropriate follow-up for CD patients.
During the conduct of this investigation, we faced some limitations. In fact, since this is a retrospective study, there was lack of information in some variables, something inherent to this type of investigation. Likewise, we had a limited number of patients with histological reassessment, and although patients needed to have started the diet for at least 12 months to be included in this study, the time interval during which they underwent endoscopic reassessment was found to be quite disparate among participants. Moreover, a selection bias may have occurred because histological reassessment is not mandatory, and possibly less compliant patients may have refused to perform this more sensitive examination for fear of the result. Additionally, the population that adhered to the revaluation endoscopy had a low complication rate, which made the prognostic assessment not feasible.
This study has several strengths, such as studying the follow-up of CD in adulthood, because the majority of the studies are still performed at pediatric age, and the fact of addressing a topic that is still very scarcely mentioned in the current literature, such as the relevance of deep remission and its clinical impact.
Surprisingly, this type of studies on CD deep remission and its possible long-term impact in adult patients are still scarce. For this reason, this study may serve as an impetus to further study this relevant subject in future investigations.
Concluding, even under GFD, normalization of the histological findings of CD is difficult to obtain and appears to be independent of most clinical and serological findings at diagnosis. Patients with less severe histological levels at diagnosis reach remission more easily, but only represent the minority of the population. This work may add value to the importance of follow-up of these patients, since a more in-depth evaluation has not yet demonstrated clinical value, as suggested by our work.
References
1 Bai JC, Ciacci C. World Gastroenterology Organisation Global Guidelines: Celiac Disease February 2017. J Clin Gastroenterol. 2017 Oct;51(9):755–68.
2 Tortora R, Zingone F, Rispo A, Bucci C, Capone P, Imperatore N, et al. Coeliac disease in the elderly in a tertiary centre. Scand J Gastroenterol. 2016 Oct;51(10):1179–83.
3 Lionetti E, Catassi C. New clues in celiac disease epidemiology, pathogenesis, clinical manifestations, and treatment. Int Rev Immunol. 2011 Aug;30(4):219–31.
4 Chou R, Bougatsos C, Blazina I, Mackey K, Grusing S, Selph S. Screening for Celiac Disease: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2017 Mar;317(12):1258–68.
5 Lohi S, Mustalahti K, Kaukinen K, Laurila K, Collin P, Rissanen H, et al. Increasing prevalence of coeliac disease over time. Aliment Pharmacol Ther. 2007 Nov;26(9):1217–25.
6 Kelly CP, Bai JC, Liu E, Leffler DA. Advances in diagnosis and management of celiac disease. Gastroenterology. 2015 May;148(6):1175–86.
7 Ludvigsson JF, Leffler DA, Bai JC, Biagi F, Fasano A, Green PH, et al. The Oslo definitions for coeliac disease and related terms. Gut. 2013 Jan;62(1):43–52.
8 Kotze LM. Celiac disease in Brazilian patients: associations, complications and causes of death. Forty years of clinical experience. Arq Gastroenterol. 2009 Oct-Dec;46(4):261–9.
9 Ciacci C, Ciclitira P, Hadjivassiliou M, Kaukinen K, Ludvigsson JF, McGough N, et al. The gluten-free diet and its current application in coeliac disease and dermatitis herpetiformis. United European Gastroenterol J. 2015 Apr;3(2):121–35.
10 Dieterich W, Ehnis T, Bauer M, Donner P, Volta U, Riecken EO, et al. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat Med. 1997 Jul;3(7):797–801.
11 Mulder CJ, Wierdsma NJ, Berkenpas M, Jacobs MA, Bouma G. Preventing complications in celiac disease: our experience with managing adult celiac disease. Best Pract Res Clin Gastroenterol. 2015 Jun;29(3):459–68.
12 Nachman F, Sugai E, Vázquez H, González A, Andrenacci P, Niveloni S, et al. Serological tests for celiac disease as indicators of longterm compliance with the gluten-free diet. Eur J Gastroenterol Hepatol. 2011 Jun;23(6):473–80.
13 Hill PG, Holmes GK. Coeliac disease: a biopsy is not always necessary for diagnosis. Aliment Pharmacol Ther. 2008 Apr;27(7):572–7.
14 Volta U, Villanacci V. Celiac disease: diagnostic criteria in progress. Cell Mol Immunol. 2011 Mar;8(2):96–102.
15 Lebwohl B, Sanders DS, Green PH. Coeliac disease. Lancet. 2018 Jan;391(10115):70–81.
16 Scoglio R, Di Pasquale G, Pagano G, Lucanto MC, Magazzù G, Sferlazzas C. Is intestinal biopsy always needed for diagnosis of celiac disease? Am J Gastroenterol. 2003 Jun;98(6):1325–31.
17 Ludvigsson JF, Bai JC, Biagi F, Card TR, Ciacci C, Ciclitira PJ, et al.; BSG Coeliac Disease Guidelines Development Group; British Society of Gastroenterology. Diagnosis and management of adult coeliac disease: guidelines from the British Society of Gastroenterology. Gut. 2014 Aug;63(8):1210–28.
18 Moreno ML, Cebolla Á, Muñoz-Suano A, Carrillo-Carrion C, Comino I, Pizarro Á, et al. Detection of gluten immunogenic peptides in the urine of patients with coeliac disease reveals transgressions in the gluten-free diet and incomplete mucosal healing. Gut. 2017 Feb;66(2):250–7.
19 Wahab PJ, Meijer JW, Mulder CJ. Histologic follow-up of people with celiac disease on a gluten-free diet: slow and incomplete recovery. Am J Clin Pathol. 2002 Sep;118(3):459–63.
20 Rubio-Tapia A, Hill ID, Kelly CP, Calderwood AH, Murray JA; American College of Gastroenterology. ACG clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol. 2013 May;108(5):656–76.
21 Moreno ML, Vazquez H, Mazure R, Smecuol E, Niveloni S, Pedreira S, et al. Stratification of bone fracture risk in patients with celiac disease. Clin Gastroenterol Hepatol. 2004 Feb;2(2):127–34.
22 Sugai E, Nachman F, Váquez H, González A, Andrenacci P, Czech A, et al. Dynamics of celiac disease-specific serology after initiation of a gluten-free diet and use in the assessment of compliance with treatment. Dig Liver Dis. 2010 May;42(5):352–8.
Statement of Ethics
The study protocol, which received ethics committee approval, conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a prior approval by the institution’s human research committee. Written, informed consent was obtained from each patient included in the study.
Disclosure Statement
The authors declare no conflicts of interest.
Funding Sources
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
* Corresponding author.
Armando Peixoto
Hospitalar Center of São João
Alameda Prof. Hernâni Monteiro
PT–4200-319 Porto (Portugal)
E-Mail armandoafp5@gmail.com
Received: August 1, 2019; Accepted after revision: November 12, 2019
Acknowledgments
The authors thanks Doctor Marco Silva for his help with statistical analysis. We are also grateful for all the availability and support of the Department of Gastroenterology of the Centro Hospitalar de Sao Joao for the execution of this project.














