Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
GE-Portuguese Journal of Gastroenterology
versão impressa ISSN 2341-4545
GE Port J Gastroenterol vol.27 no.4 Lisboa ago. 2020
https://doi.org/10.1159/000504719
CLINICAL CASE STUDY
Gastrointestinal Tuberculosis Mimicking Crohn’s Disease
Tuberculose gastrointestinal simulando doença de Crohn
Maria Ana Rafaela, Luísa Martins Figueiredoa, Ana Maria Oliveiraa, Mariana Nuno Costaa, Rita Theias Mansob, Alexandra Martinsa
aGastroenterology Department, Hospital Professor Doutor Fernando Fonseca, Amadora, Portugal; bPathologic Anatomy Department, Hospital Professor Doutor Fernando Fonseca, Amadora, Portugal
* Corresponding author.
ABSTRACT
We present the case of a 24-year-old woman with complaints of abdominal pain, bloody diarrhea, and weight loss for 3 months. An outpatient colonoscopy revealed scattered ulcers, suggestive of Crohn’s disease (CD). Histopathology also favored the diagnosis of CD. However, after admission to our hospital for further investigation, a chest radiograph revealed pulmonary cavitations. A computed tomography scan suggested the diagnosis of active pulmonary tuberculosis (TB). Therefore, a bronchofibroscopy, a total colonoscopy with ileoscopy, and an upper endoscopy were performed. Not only were acid-fast bacilli present in both bronchoalveolar lavage fluid and gastric juice, but also in colonic biopsies. A complete resolution of gastrointestinal symptoms was achieved 2 weeks after starting anti-TB drugs.
Keywords: Gastrointestinal tuberculosis, Crohn’s disease, Inflammatory bowel disease
RESUMO
Apresentamos o caso de uma mulher de 24 anos com queixas de dor abdominal, diarreia sanguinolenta e perda ponderal com 3 meses de evolução. Uma colonoscopia de ambulatório revelou, de forma descontínua, úlceras sugestivas de doença de Crohn (DC). Também a histologia era sugestiva de DC. Contudo, após admissão no nosso hospital, uma radiografia de tórax evidenciou cavitações pulmonares. A tomografia computorizada sugeriu o diagnóstico de tuberculose (TB) pulmonar ativa. Por esse motivo, foram realizadas broncofibroscopia, endoscopia digestiva alta e colonoscopia total com ileoscopia. A pesquisa de bacilos ácido-álcool resistentes foi positiva não só no lavado broncoalveolar e no suco gástrico, mas também nas biópsias do cólon. Verificou-se uma resolução completa das queixas gastrointestinais 2 semanas após iniciar antibacilares.
Palavras-Chave: Tuberculose intestinal, Doença de Crohn, Doença inflamatória intestinal
Introduction
Gastrointestinal tuberculosis (TB) is a diagnostic challenge, as the nonspecific features of the disease may lead to diagnostic delays and development of complications. It is regarded as a great mimicker of other pathologies, such as Crohn’s disease (CD). It accounts for 1–3% of all TB cases and 11% of extrapulmonary TB cases [1]. A high index of suspicion is an important factor for an early diagnosis [2]. In case of misdiagnosis of gastrointestinal TB, unnecessary antibacillary therapy poses a risk of toxicity and delays the treatment of the primary disease. In contrast, steroids, which are used for CD, can be disastrous for Mycobacterium tuberculosis diffusion [3].
Concomitant pulmonary TB, ascites, night sweats, involvement of fewer than four segments of the bowel, patulous ileocecal valve, transverse ulcers, scars, or pseudopolyps strongly indicate gastrointestinal TB. Bloody stools, perianal signs, chronic diarrhea, extraintestinal manifestations, anorectal lesions, longitudinal ulcers, and a cobblestone appearance are all suggestive of CD [1].
Case Report
We present the case of a Portuguese 24-year-old woman with abdominal pain, bloody diarrhea, and weight loss for 3 months. She denied fever, sweating, and respiratory or urinary symptoms. Her past medical history was irrelevant. She underwent an outpatient colonoscopy, which revealed scattered ulcers from the rectum to the transverse colon, where the examination was interrupted due to the severity of the lesions (Fig. 1a, b).
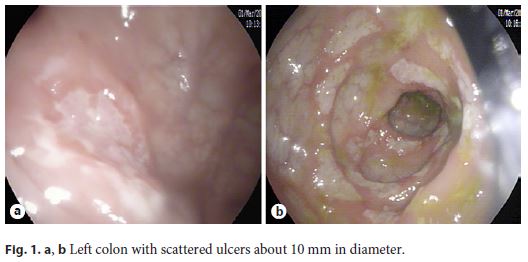
Histopathology revealed architectural distortion, with an inflammatory process with lymphoplasmacytic infiltration and cryptitis, suggestive of CD. Immunohistochemistry for Cytomegalovirus and histochemistry for acid-fast bacillus (AFB) in Ziehl- Neelsen stain were negative.
The patient was referred to our hospital for further diagnosis and treatment. At admission, she had a normocytic/normochromic anemia (Hg 11.7 g/dL, MCV 89 fL), without leukocytosis, but with elevated CRP (8.02 mg/dL) and ESR (57 mm/h). She had folic acid deficiency, with normal iron and vitamin B12 levels. Human immunodeficiency virus, hepatitis C virus, cytomegalovirus, Epstein Barr virus, and herpes simplex virus serologies were negative, and she was immune to hepatitis B virus. Stool cultures and Clostridium difficile toxin were negative. An abdominal ultrasound revealed circumferential parietal thickening of the ascending colon and increased echogenicity of the adjacent adipose planes.
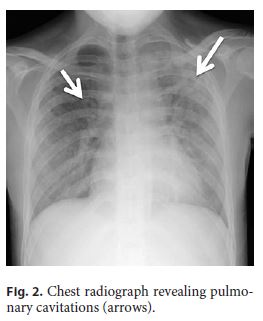
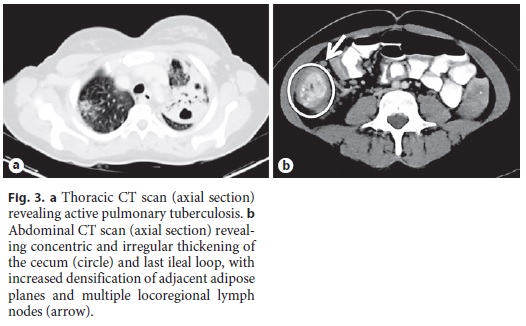
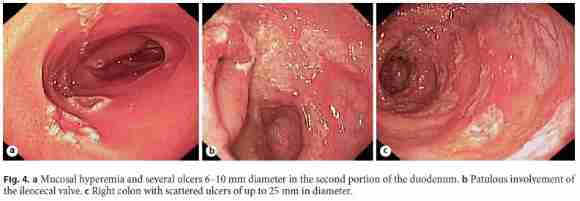
She was started on salicylates, ciprofloxacin, and metronidazole, with partial improvement in the frequency of bowel movements with bleeding resolution. However, because her admission chest radiograph revealed pulmonary cavitations (Fig. 2), a thoracic, abdominal, and pelvic computed tomography (CT) scan was performed, suggesting the diagnosis of active pulmonary TB (Fig. 3a). It also revealed a concentric and irregular thickening of the cecum, extending to the last ileal loop. There was an increased densification of adjacent adipose planes and multiple locoregional nonnecrotic lymph nodes (Fig. 3b). With these findings, a bronchofibroscopy, an upper endoscopy, and a total colonoscopy with ileoscopy under anesthesia were performed. Macroscopically, the bronchofibroscopy was normal. However, the upper endoscopy revealed several ulcers 6–10 mm in diameter in the second portion of the duodenum (Fig. 4a). Ulcers of up to 25 mm in diameter were also found in the terminal ileum and colon, mainly on the right side (Fig. 4b, c).
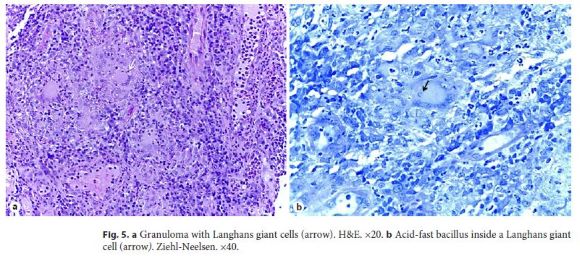
Because acid-fast staining was both positive in the gastric juice and bronchoalveolar lavage, she was immediately transferred to a negative room pressure. Biopsies from the right colon revealed Langhans giant cells with an AFB inside one of these cells (Fig. 5a, b). She started rifampicin, pyrazinamide, isoniazid, and ethambutol, with complete resolution of gastrointestinal symptoms after 2 weeks of treatment. Only later was her interferon-γ release assay (IGRA) available and it was positive.
Discussion and Conclusion
Both CD and gastrointestinal TB are characterized by chronic granulomatous inflammation in the gastrointestinal tract, with clinical features including abdominal pain, recurrent partial intestinal obstruction, fever, anorexia, weight loss, chronic diarrhea, hematochezia, perianal disease, and extraintestinal manifestations like arthralgia, aphthous ulcers, and dermatologic and ocular manifestations. Although longer duration of disease, perianal disease, and extraintestinal manifestations favored a diagnosis of CD, and fever and night sweats favored a diagnosis of TB, none of these features are specific [4].
The most common site of gastrointestinal TB is an ileocecal location, followed by the jejunum and colon. The esophagus, stomach, and duodenum are rarely involved. The most common symptom of jejunal and ileocecal TB is bowel obstruction, whereas abdominal pain and change in bowel habits are the most frequent symptoms in colonic TB. However, isolated involvement of the colon is only present in about 10% of gastrointestinal TB. The cecum is the most common site of colonic involvement, but it is usually in contiguous involvement with the terminal ileum [4].
Among the endoscopic features, the presence of longitudinal ulcers, aphthous ulcers, cobblestoning, and skip lesions are more common in CD, whereas the presence of transverse ulcers and patulous ileocecal valve are more common in gastrointestinal TB. Concerning pathology findings, architectural abnormalities such as crypt distortion/branching/shortening and irregular mucosal surface, though more common in CD, are also seen in gastrointestinal TB. Granulomas are more frequently seen in gastrointestinal TB and are usually larger, confluent, dense, located in submucosa, and characterized by central caseation, which is almost pathognomonic of TB. Since intestinal TB is a paucibacillary disease, positive AFB staining in mucosal biopsies has a sensitivity of only 2.7–37.5%. The sensitivity of AFB culture varies from 19 to 70% and PCR for AFB in endoscopic biopsies is not exclusive for gastrointestinal TB. CT findings more commonly seen in patients with CD are long-segment involvement, skip lesions, and presence of comb sign. On the other hand, lymph nodes larger than 1 cm favor gastrointestinal TB. It is also important to emphasize that chest radiograph evidences active/healed pulmonary TB in only up to 25% of patients with gastrointestinal TB [4].
To help differentiate CD from gastrointestinal TB, predictive scores have been suggested, although external validation for various populations is still lacking. However, these studies emphasize predictive features for each disease. Concerning clinical features, diarrhea, hematochezia, presence of perianal disease, and extraintestinal manifestations significantly favored CD, whereas fever, night sweats, lung involvement, and ascites significantly favored gastrointestinal TB. Inflammatory markers, such as CRP or ESR, are inconsistently included in these models due to the disparity of results. The endoscopic, radiologic, and pathologic findings previously discussed here are also included in these predictive models [5, 6].
Most patients with gastrointestinal TB respond well to antibacillary therapy, which must be maintained for at least 6 months. With resolution of gastrointestinal symptoms, an endoscopy to confirm mucosal healing is not necessary [2].
This case report evidences the importance of a high level of suspicion of gastrointestinal TB when evaluating a patient with colonic ulcers favoring a CD diagnosis, even in a nonendemic country. Although the patient in this case did not have symptoms suggestive of active pulmonary TB, later we discovered that she had had previous contact with a bacilliferous relative, having already been treated for latent TB.
We would also like to emphasize that the patient in the presented case had some clinical manifestations favoring CD diagnosis, such as hematochezia and absence of fever, night sweats, or respiratory complaints. The patient also had duodenal and distal colonic involvement, with crypt distortion and mesenteric fat hypertrophy, favoring CD. However, chest radiograph and CT scan were suggestive of TB. Although nonnecrotic, the presence of multiple locoregional lymph nodes also favored the diagnosis of gastrointestinal TB.
Immunocompromised patients are particularly prone to present atypical forms of TB, such as gastrointestinal and disseminated TB. Therefore, it is widely recommended to screen for latent TB in all patients who are expected to start immunosuppressive therapy, such as patients with CD. Most of the overt TB cases are due to reactivation of latent infection, occurring approximately 12 weeks after the initial exposure to infliximab, usually within the first 6 months of therapy. This drug is associated with a 5-fold increased risk of reactivation of TB in the first 52 weeks after initiation of therapy [7]. Although it is standard of care to perform a chest radiograph and an IGRA test before starting immunosuppressive therapy in CD, the sensitivity of these tests for gastrointestinal TB is approximately 25 and 80%, respectively [4]. Additionally, IGRA results often take time and can be positive in either active or latent TB. Therefore, it is fundamental to obtain a complete past medical history, previous contacts with infectious diseases, and travelling history when evaluating a patient with a presumptive diagnosis of inflammatory bowel disease.
References
1 Ma JY, Tong JL, Ran ZH. Intestinal tuberculosis and Crohn’s disease: challenging differential diagnosis. J Dig Dis. 2016 Mar;17(3):155–61.
2 Debi U, Ravisankar V, Prasad KK, Sinha SK, Sharma AK. Abdominal tuberculosis of the gastrointestinal tract: revisited. World J Gastroenterol. 2014 Oct;20(40):14831–40.
3 Zhao XS, Wang ZT, Wu ZY, Yin QH, Zhong J, Miao F, et al. Differentiation of Crohn’s disease from intestinal tuberculosis by clinical and CT enterographic models. Inflamm Bowel Dis. 2014 May;20(5):916–25.
4 Kedia S, Das P, Madhusudhan KS, Dattagupta S, Sharma R, Sahni P, et al. Differentiating Crohn’s disease from intestinal tuberculosis. World J Gastroenterol. 2019 Jan;25(4):418–432.
5 Jung Y, Hwangbo Y, Yoon SM, Koo HS, Shin HD, Shin JE, et al. Predictive factors for differentiating between Crohn’s disease and intestinal tuberculosis in Koreans. Am J Gastroenterol. 2016 Aug;111(8):1156–64.
6 Limsrivilai J, Shreiner AB, Pongpaibul A, Laohapand C, Boonanuwat R, Pausawasdi N, et al. Meta-analytic Bayesian model For differentiating intestinal tuberculosis from Crohn’s disease. Am J Gastroenterol. 2017 Mar;112(3):415–27.
7 Carvalho LP, Túlio MA, Rodrigues JP, Bana E Costa T, Chagas C. Miliary tuberculosis in a Crohn’s disease patient: the risk beyond the screening. GE Port J Gastroenterol. 2018 Dec;26(1):64–9.
Statement of Ethics
The authors have no ethical conflicts to disclose.
Disclosure Statement
The authors have no conflicts of interest to declare.
* Corresponding author.
Maria Ana Rafael
Gastroenterology Department, Hospital Professor Doutor Fernando Fonseca
IC 19, PT–2720-276 Amadora (Portugal)
E-Mail maria.monteiro.rafael@gmail.com
Received: July 20, 2019; Accepted after revision: October 27, 2019














