Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
GE-Portuguese Journal of Gastroenterology
versão impressa ISSN 2341-4545
GE Port J Gastroenterol vol.27 no.1 Lisboa fev. 2020
https://doi.org/10.1159/000501404
ORIGINAL ARTICLE
How Is Endoscopic Submucosal Dissection for Gastrointestinal Lesions Being Implemented? Results from an International Survey
Como é que a disseção endoscópica da submucosa está a ser implementada no tratamento de lesões gastrointestinais? Resultados de um questionário internacional
Miguel Araújo-Martinsc, Pedro Pimentel-Nunesa–c, Diogo Libânioa,b, Marta Borges-Canhac, Mário Dinis-Ribeiroa,b
aDepartment of Gastroenterology, Portuguese Oncology Institute of Porto, Porto, Portugal; bCenter for Research in Health Technologies and Information Systems (CINTESIS), Faculty of Medicine, University of Porto, Porto, Portugal; cDepartment of Surgery and Physiology, Faculty of Medicine, University of Porto, Porto, Portugal
* Corresponding author.
ABSTRACT
Background and Study Aim: Superficial gastrointestinal (GI) neoplasms can be treated with endoscopic mucosal resec-tion (EMR) and/or endoscopic submucosal dissection (ESD). These techniques are widely used in Eastern countries; how-ever, its use in the West is limited. The aim of this study was to evaluate the current implementation of ESD in Western countries. Methods: Western endoscopists (n = 279) who published papers related to EMR/ESD between 2005 and 2017 were asked to complete an online survey from Decem-ber 2017 to February 2018. Results: A total of 58 endosco-pists (21%) completed the survey. Thirty performed ESD in the esophagus (52%), 45 in the stomach (78%), 36 in the co lorectum (62%), and 6 in the duodenum (10%). The median total number of lesions ever treated per endoscopist was 190, with a median number per endoscopist in 2016 of 41 (7 [IQR 1–21], 6 [IQR 4–16], and 28 [5–63] in the esophagus, in the stomach, and in the colon and rectum, respectively). En bloc resection rates were 97% in the esophagus, 95% in the stomach, and 84% in the colorectum. Complete resection (R0) was achieved in 88, 91, and 81%, respectively. Curative rates were 69, 70, and 67%, respectively. Major complica-tions (perforation or delayed bleeding) occurred more often in colorectal ESD (12 vs. 6% in the esophagus and 7% in the stomach). In the upper GI tract, the majority of resected le-sions were intramucosal adenocarcinoma (59% in the esoph-agus; 47% in the stomach), while in the colorectum the ma-jority were adenomas (59%). Conclusion: ESD seems to be performed by a large number of centers and endoscopists. Our results suggest that ESD is being successfully imple-mented in Western countries, achieving a good rate of effi-cacy and safety according to European guidelines.
Keywords: Endoscopic submucosal dissection, Survey, Gastrointestinal superficial lesions
RESUMO
Introdução e objetivos: As lesões superficiais gastrointestinais podem ser tratadas por musectomia (EMR) e/ou disseção endoscópica da submucosa (ESD). Estas técnicas são usadas frequentemente nos países asiáticos, mas a experiência é mais limitada nos países ocidentais. O objetivo deste estudo foi avaliar a implementação atual da ESD nos países ocidentais. Métodos: Gastroenterologistas ocidentais (n = 279) com artigos publicados entre 2005 e 2017 relacionados com EMR/ESD foram solicitados a preencher um questionário online, no período de Dezembro 2017 até Fevereiro 2018. Resultados: Um total de 58 gastroenterologistas (21%) completou o inquérito. Trinta realizaram ESD esofágica (52%); 45 gástrica (78%); 36 coloretal (62%); e 6 duodenal (10%). A mediana do número total de lesões ressecadas por endoscopista foi 190, sendo que, em 2016, a mediana de lesões tratadas por cada gastroenterologista foi 41 (7 [IQR 1–21] no esófago, 6 [IQR 4–16] no estômago e 28 [5–63] no cólon e reto). A taxa de ressecção em bloco foi de 97% nas lesões esofágicas; 95% nas lesões gástricas e 84% nas lesões coloretais, com uma proporção de casos R0 de 88, 91 e 81%, respetivamente. A taxa de casos curados foi de 69, 70 e 67%, respetivamente. A taxa de complicações graves (perfuração e hemorragia tardia) foi maior na ESD coloretal (12% dos casos vs. 6% no esófago e 7% no estômago). A maioria das lesões esofagogástricas eram adenocarcinomas intramucosos (59% no esófago; 47% no estômago), enquanto as lesões coloretais eram maioritariamente adenomas (59%). Conclusões: Este estudo mostra uma disseminação da ESD na europa por um maior número de centros e gastrenterologistas. Os nossos resultados sugerem uma utilização e eficácia global de acordo com as recomendações europeias.
Palavras-chave: Disseção endoscópica da submucosa, Questionário,·Lesões superficiais gastrointestinais
Introduction
Superficial gastrointestinal (GI) neoplastic lesions with minimal risk of lymph node metastasis can be treated with minimally invasive endoscopic procedures, namely with endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) [1].
EMR was first described in Japan in the early 1990s, and it is suitable for treating a majority of superficial esophageal and colorectal lesions [1]. Still, if lesions are larger than 15–20 mm or non-lifting, it is generally not possible to remove them en bloc, possibly leading to high local recurrence rates [2]. ESD was developed years later to provide en bloc R0 resections regardless of the size of tumor and a more precise histopathological assessment, even though it is considered technically more difficult to perform and associated with a higher complication rate [2–4].
Although ESD is widely used in Eastern countries, its use in the West is limited, and in some cases, treatment still requires surgery even for initial stages [5]. Nonetheless, since it is proven that this resection technique is equally effective and potentially safer than surgery in the treatment of early GI cancers, ESD is progressively gaining more attention by Western endoscopists [5–9].
Our previous study in 2010, focusing on how ESD was being established in Europe for treating gastric lesions, suggested that this technique was performed at few centers, with most endoscopists performing a low number of procedures [10]. It also suggested that many patients could be endoscopically treated with comparable en bloc and R0 resection rates to the Eastern ones at the expense of slightly higher number of adverse events [10].
A growing number of Western series have been published since then and they seem to report similar results compared to the Eastern ones, apart from colorectal ESD [5]. The aim of this study was to evaluate the current implementation of ESD in Western countries, 8 years after our previous study.
Methods and Participants
Study Design and Selection of Participants
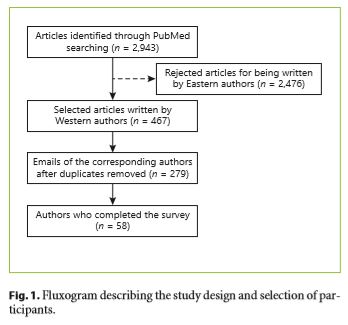
A MedLine search (through PubMed) was performed to identify published articles related to ESD using the following query: “endoscopic mucosal resection” (Title) OR “EMR” (Title) OR “endoscopic submucosal dissection” (Title) OR “ESD” (Title) AND (“2005/01/01” [PDAT]: “2017/07/03” [PDAT]). The authors of the 2,943 retrieved articles were identified. After excluding Eastern authors, 279 papers were selected and the corresponding author was contacted by E-Mail and asked to answer a survey built using Google’s Form (platform by Google USA). In case of non-response, 2 additional invitations were sent in a 3-month period. Participants were asked to fill it as close as possible to their own clinical/their department’s practice. All responses were anonymous.
The answers of 58 western endoscopists whose research, published between 2005 and 2017, was related to EMR or submucosal dissection were selected to this cross-sectional study (Fig. 1).
Survey Characterization
The survey (Appendix 1) was designed to evaluate the use of ESD technique in 3 different main GI sectors (esophagus, stomach, colon, and rectum) and included 5 main groups of questions:
(a) General data – assessed whether participants were performing ESD or not and, if yes, how many cases were they treating with this technique (ever and in the last year, 2016)
(b) Pre-procedure – assessed whether participants were doing radiological or endosonographic evaluation before endoscopic resection and, if yes, what type of examinations did they perform
(c) Procedure – assessed how did the participants characterize the lesions resected (in terms of localization, size, Paris classification [11], histological grade) and how did they perform the ESD technique (instruments, dissection techniques, types of submucosal injection solutions, and strategies for prevention of post-ESD bleeding used)
(d) Post-procedure – assessed whether participants were doing a second-look endoscopy after performing ESD, how did they manage their patients after ESD (acid suppression strategy andoral feeding protocol) and how their outcomes were, regarding en bloc resection and completeness of resection
(e) Follow-up – assessed local and metachronous recurrence rates, the proportion of surgeries due to ESD complications/noncurative disease and the follow-up protocol after endoscopic resection.
It also included 1 question to assess whether participants were performing ESD in the duodenum or not. However, we did not extend the survey as in other GI sectors since ESD is not recommended in duodenum by European guidelines [5].
En bloc resection; R0, R1, and Rx resection; frank and micro perforation, major acute (during the procedure) and delayed bleeding; and local and metachronous recurrence were defined according to European Guidelines [5].
Statistical Analysis
Data were exported from Google’s Form and prepared for statistical analysis. Given the number of answers and the characteristics of the survey, statistical analysis was descriptive. We decided to summarize our variables using absolute and relative frequencies, weighted means or medians. We used SPSS software version 23 (SPSS Inc., Chicago, IL, USA) to descriptively summarize our data.
Results
Description of the Participants and Procedures
Of the 279 contacted physicians, 58 completed the survey (21%) and only their responses were included in this study.
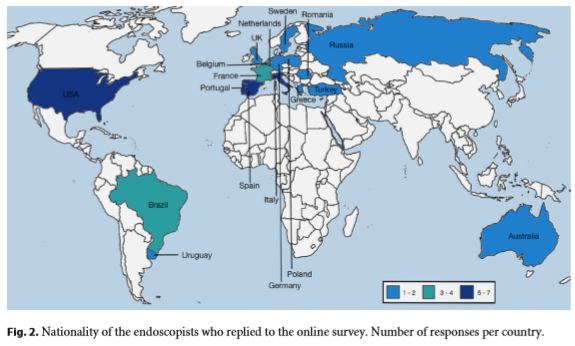
While 64% of the endoscopists’ answers reflected their own experience, 36% reproduced their department’s dayto- day practice. Table 1 summarizes the main results concerning participants, resected lesions, outcomes, complications, and follow-up. Figure 2 shows the nationality of the physicians who answered the online survey.
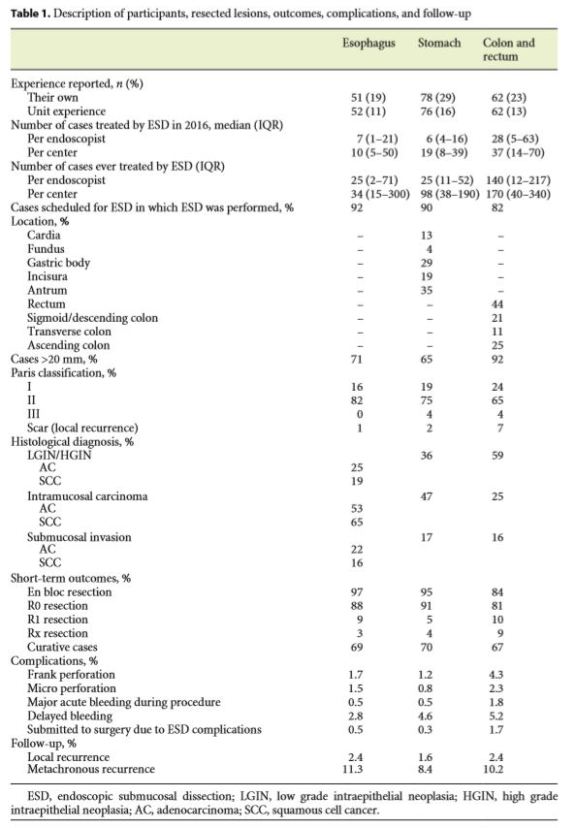
Of the 58 participants, 50 (86%) reported that they performed ESD at least in 1 GI location. Thirty endoscopists performed ESD in the esophagus (52%), 45 in the stomach (78%), 36 in the colon and rectum (62%). Only 6 participants performed ESD in the duodenum (10%).
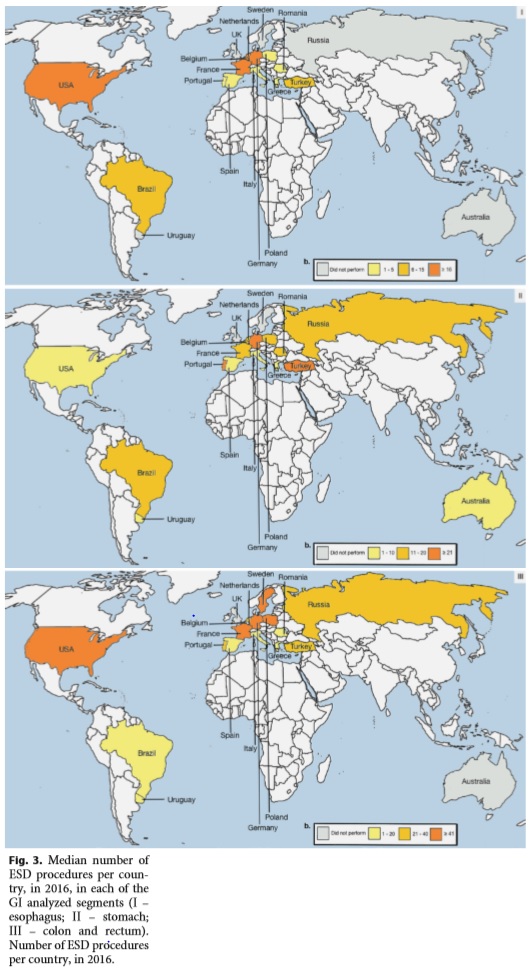
The median number of lesions ever resected by each endoscopist was 25 in the esophagus (IQR 2–71), 25 in the stomach (IQR 11–52), and 140 in the colon and rectum (IQR 12–217). Figure 3 shows the median number of ESD procedures per country, in 2016, in each of the GI analyzed segments.
Description of the Resected Lesions and Outcomes
Esophagus
The median minimum diameter of the resected tumors was 12 mm (IQR 10–17.5) and the median maximum was 70 mm (IQR 40–100).
Concerning the histological analysis of the esophageal resected lesions, 58% were classified as Barrett’s esophagus-associated lesions and 42% as squamous cell lesions.
In both types of esophageal carcinomas – Barrett’s esophagus-associated adenocarcinoma (AC) and squamous cell cancer – the majority of the lesions were classified as intramucosal carcinomas (53 and 65%, respectively), followed by intra-epithelial neoplasias (3 and 2% were classified as low-grade dysplasia, respectively; 22 and 17% were classified as high-grade dysplasia, respectively).
Altogether, 69% of the lesions treated with ESD technique were approached as curative cases, with 12% of patients being submitted to additional treatment (surgery, chemotherapy, and/or radiotherapy) after ESD.
Stomach
The median minimum and maximum diameter of resected lesions was 10 mm (IQR 10–15) and 60 mm (IQR 41.25–80), respectively.
At post-resection histology, lesions were more frequently classified as intramucosal ACs (47%), followed by intraepithelial neoplasias (26% high-grade dysplasia and 10% low-grade dysplasia).
The resection was considered curative in 70% of the lesions. However, in 24% of the cases, patients were submitted to surgery despite ESD due to non-curative resection.
Colon and Rectum
In this GI domain, lesions measured between 15 mm (IQR 10–20) and 70 mm (IQR 6–270).
In the colon and rectum, intraepithelial neoplasias were more common than intramucosal ACs (59 vs. 25%, respectively). Low-grade dysplasia was found in 25% of the cases, while 34% presented with high-grade dysplasia.
ESD technique managed to be curative in 67% of the lesions. In 11% of the cases, patients were submitted to surgery due to non-curative resection.
Description of ESD Technique
Table 2 summarizes the main results concerning ESD technique.
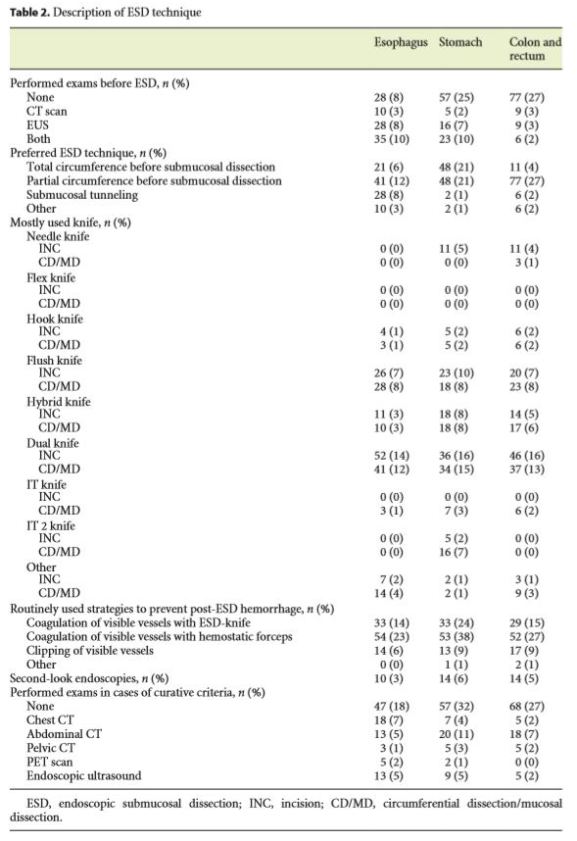
Saline with methylene blue was the preferred submucosal injection in every location. Dual knife was the most used knife in incision and in circumferential/ mucosal dissection (> 34%) in every location, followed by Flush and Hybrid-knife.
After gastric ESD, the most used acid suppression strategy was PPI perfusion during 48 h, switched to oral intake during 2–8 weeks (53%). In the absence of complications, oral feeding started more frequently 1 day after ESD, in every location (58% in esophageal ESD; 66% in stomach ESD; 60% in colorectal ESD). In colon and rectum, the majority of the physicians did not prescribe antibiotics (71%).
Discussion
To our knowledge this is the first study evaluating ESD implementation in other GI sectors aside from the stomach, in the West. This study suggests that not only the total number of physicians who perform ESD is increasing in Western countries, but also that Western endoscopists are achieving similar results to those in the East, both in terms of efficacy and safety in the treatment of early GI neoplastic lesions.
This study has some limitations. On the one hand, our study is based only on reports of endoscopists who write and publish papers. These physicians are more motivated and may have better results. Additionally, since a part of all ESDs (performed by physicians who were not contacted) were not included, a selection bias might be present. On the other hand, we cannot exclude the possibility of having Eastern endoscopists working in the Western countries. Furthermore, since each endoscopist declares his own/his departments’ outcomes and we also do not know how many centers/endoscopists have their performed cases indexed in databases, this study possibly leads to a reporting bias. Moreover, the response rate is low – approximately, only 1 in 5 of the recipient endoscopists completed the survey. All of these limitations can overestimate the true efficacy and safety of ESD technique in the West. Nevertheless, all of them are inherent to the study design and our participation rate is even higher than most survey studies.
In our previous European survey performed in 2010, 30% of the endoscopists reported that they performed gastric ESD. In 2018, considering European participants only, 93% reported that they performed ESD in the stomach [10]. Table 3 compares the main results of both studies.
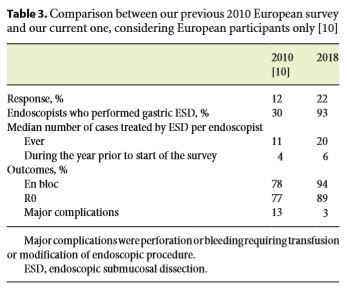
Some particularities should be mentioned about this study. First, this new survey was designed to evaluate the implementation of ESD technique not only in the stomach, but also in others GI sectors, namely the esophagus and the colon and rectum. Second, it includes more Western countries apart from European ones, which enables a more global and complete perspective of the ESD’s dissemination. Third, we obtained a higher response rate in this study, which increased from 12% (in the previous one) to 21% (22%, considering European participants only). Although it is still a low percentage, as mentioned before, it was higher than expected. This increase may be partially explained by the current importance of this theme around Western endoscopy community.
In the esophagus, approximately 60% of the lesions were classified as Barrett’s esophagus-associated lesions and 40% as squamous cell lesions, which is consistent with the higher incidence of Barrett’s esophagus in Western countries [12]. Since our esophageal ESD outcomes include 2 types of esophageal superficial lesions, we could not compare them to the majority of recent studies, which focus only on Barrett’s esophagus-associated neoplasias or only on squamous cell lesions. Nonetheless, considering European guidelines, our results suggest that esophageal ESD is being safely performed with good resection rates [5]. However, given the high number of esophageal ESDs, ESD is probably being performed for some Barrett´s lesions that could be treated by EMR. As a matter of fact, a recent European randomized controlled trial, consistent with 2015 European guidelines, suggests that ESD does not seem to have any clinical advantage, compared to EMR, for excision of the majority of visible early Barrett’s esophagus lesions [5, 13].
In the stomach, our results suggest that ESD is being safely performed with good resection rates too. These results are better than our previous results (Table 3) and are consistent with a systematic review with meta-analysis published in 2016 related to gastric ESD [14]. Interestingly, gastric ESD only managed to be curative in 70% of the lesions, which is lower than what was reported by the meta-analysis mentioned above (86% with a 95% CI 83–89%) [14]. Probably, since en-bloc and R0 rates are similar to western series, this means that Western endoscopists are resecting some advanced gastric lesions already with a high risk of deep submucosal invasion, and probably patient selection could be improved [15, 16].
In colon and rectum, although ESD is performed by a lower percentage of endoscopists compared to the stomach, the median number of cases treated by this technique is higher. In fact, in absolute terms, colorectal ESD is associated with the highest volume of cases per endoscopist/ per center. This may be explained by the fact that the incidence of colorectal cancer is the highest of all GI neoplasms [12] and that the classic indication of early gastric lesions in the East are not frequently observed in most Western countries.
In this study, colorectal ESD had the lowest resection rate and the highest adverse event rate of the 3 GI sectors. Contrary to the stomach, where outcomes were similar to recent data, in the colon and rectum, outcomes were consistently lower. Only R0 resection rate was comparable to a recent meta-analysis published in 2016 [17]. Moreover, contrary to the upper GI, intraepithelial neoplasias (dysplasia) were more common than carcinomas (approximately 60% non-invasive/benign neoplasias versus 40% invasive/malign neoplasias). This point is very important to discuss as colorectal ESD is being performed more often than necessary.
Actually, colorectal ESD is controversial. Part of the scientific community advocates that ESD should be the standard number-one option to treat all large colorectal lesions, since it is associated with superior en bloc and R0 resection rates and lower recurrence rates, compared to EMR [18–24]. On the contrary, some argue that it is not cost-effective to treat all these lesions with ESD, as it is more time-consuming, has a higher adverse event rate, is technically more difficult and, more importantly, the majority of the lesions can be effectively and more safely removed by EMR [25–28]. Until the present moment, there are no randomized controlled trials comparing ESD with EMR. Therefore, considering that these techniques are currently used for different indications, a comparison between them, based on retrospective studies, is a difficult task due to selection bias [29].
Regarding safety profile, the general outcome improvement is consistent with a national survey conducted in France, during 2017. This study showed that there has been an improvement over time of en bloc resection rate, which has increased from 77.1 to 91.7% (p < 0.001), and a decrease over time in complication rate, which went from 29.2 to 14.1% (p < 0.001), since the implementation of ESD technique in the country [30]. Delayed bleeding remains one of the main complications of ESD. Prophylactic coagulation of visible vessels and use of an acid suppression strategy (proton pump inhibitors primarily) are important to reduce the risk of delayed bleeding [31, 32]. Nonetheless, even with these strategies, evidence suggests that there is still approximately 5% delayed bleeding rate associated with gastric ESD, for example, consistent with our rates [31–33]. Physicians are opting now to perform second look endoscopies, which is also consistent with current evidence that suggests that the majority of the patients submitted to ESD might not benefit from second look endoscopy [31–33].
In conclusion, ESD seems to be performed in a safe and effective way by a large number of centers and endoscopists in the Western world. Our results suggest widespread ESD implementation in Western countries, with good rates of efficacy and safety according to European guidelines, with the exception of colorectal ESD that could be used more often than necessary. It is necessary to establish adequate training programs for Western trainees, since a traditional Eastern stepwise mentor/apprentice approach may not always be suitable [5]. In addition, standardization of the ESD technique and establishment of criteria for its use are essential, to accomplish similar results to Eastern series.

References
1 Gotoda T, Jung HY. Endoscopic resection (endoscopic mucosal resection/ endoscopic submucosal dissection) for early gastric cancer. Dig Endosc. 2013 Mar;25Suppl 1:55–63. [ Links ]
2 Lang GD, Konda VJ, Siddiqui UD, Koons A, Waxman I. A single-center experience of endoscopic submucosal dissection performed in a Western setting. Dig Dis Sci. 2015 Feb;60(2):531–6. [ Links ]
3 Białek A, Pertkiewicz J, Karpińska K, Marlicz W, Bielicki D, Starzyńska T. Treatment of large colorectal neoplasms by endoscopic submucosal dissection: a European singlecenter study. Eur J Gastroenterol Hepatol. 2014 Jun;26(6):607–15. [ Links ]
4 Lian J, Chen S, Zhang Y, Qiu F. A meta-analysis of endoscopic submucosal dissection and EMR for early gastric cancer. Gastrointest Endosc. 2012 Oct;76(4):763–70. [ Links ]
5 Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, Repici A, Vieth M, De Ceglie A, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015 Sep;47(9):829–54. [ Links ]
6 Catalano F, Trecca A, Rodella L, Lombardo F, Tomezzoli A, Battista S, et al. The modern treatment of early gastric cancer: our experience in an Italian cohort. Surg Endosc. 2009 Jul;23(7):1581–6. [ Links ]
7 Deprez PH, Bergman JJ, Meisner S, Ponchon T, Repici A, Dinis-Ribeiro M, et al. Current practice with endoscopic submucosal dissection in Europe: position statement from a panel of experts. Endoscopy. 2010 Oct;42(10):853–8. [ Links ]
8 Pimentel-Nunes P, Mourao F, Veloso N, Afonso LP, Jácome M, Moreira-Dias L, et al. Long-term follow-up after endoscopic resection of gastric superficial neoplastic lesions in Portugal. Endoscopy. 2014 Nov;46(11):933– 40. [ Links ]
9 Libanio D, Braga V, Ferraz S, Castro R, Lage J, Pita I, et al. Prospective comparative study of endoscopic submucosal dissection and gastrectomy for early neoplastic lesions including patients’ perspectives. Endoscopy. 2019 Jan;51(1):30–39.
10 Ribeiro-Mourao F, Pimentel-Nunes P, Dinis-Ribeiro M. Endoscopic submucosal dissection for gastric lesions: results of an European inquiry. Endoscopy. 2010 Oct;42(10):814–9. [ Links ]
11 Participants in the Paris Workshop. The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003 Dec;58(6 Suppl):S3–43. [ Links ]
12 Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Pineros M, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019 Apr;144(8):1941–53. [ Links ]
13 Terheggen G, Horn EM, Vieth M, Gabbert H, Enderle M, Neugebauer A, et al. A randomised trial of endoscopic submucosal dissection versus endoscopic mucosal resection for early Barrett’s neoplasia. Gut. 2017 May;66(5):783–93.
14 Akintoye E, Obaitan I, Muthusamy A, Akanbi O, Olusunmade M, Levine D. Endoscopic submucosal dissection of gastric tumors: A systematic review and meta-analysis. World J Gastrointest Endosc. 2016 Aug; 8(15):517–32. [ Links ]
15 Libânio D, Pimentel-Nunes P, Afonso LP, Henrique R, Dinis-Ribeiro M. Long-Term Outcomes of Gastric Endoscopic Submucosal Dissection: Focus on Metachronous and Non-Curative Resection Management. GE Port J Gastroenterol. 2017 Jan; 24(1): 31–9. [ Links ]
16 Probst A, Schneider A, Schaller T, Anthuber M, Ebigbo A, Messmann H. Endoscopic submucosal dissection for early gastric cancer: are expanded resection criteria safe for Western patients? Endoscopy. 2017 Sep;49(9):855–65. [ Links ]
17 Akintoye E, Kumar N, Aihara H, Nas H, Thompson CC. Colorectal endoscopic submucosal dissection: a systematic review and meta-analysis. Endosc Int Open. 2016 Oct;4(10):E1030–44. [ Links ]
18 Arezzo A, Passera R, Marchese N, Galloro G, Manta R, Cirocchi R. Systematic review and meta-analysis of endoscopic submucosal dissection vs endoscopic mucosal resection for colorectal lesions. United European Gastroenterol J. 2016 Feb;4(1):18–29. [ Links ]
19 Backes Y, Moons LM, van Bergeijk JD, Berk L, Ter Borg F, Ter Borg PC, et al. Endoscopic mucosal resection (EMR) versus endoscopic submucosal dissection (ESD) for resection of large distal non-pedunculated colorectal adenomas (MATILDA-trial): rationale and design of a multicenter randomized clinical trial. BMC Gastroenterol. 2016 May;16(1):56. [ Links ]
20 Boda K, Oka S, Tanaka S, Nagata S, Kunihiro M, Kuwai T, et al. Clinical outcomes of endoscopic submucosal dissection for colorectal tumors: a large multicenter retrospective study from the Hiroshima GI Endoscopy Research Group. Gastrointest Endosc. 2018 Mar;87(3):714–22. [ Links ]
21 Chao G, Zhang S, Si J. Comparing endoscopic mucosal resection with endoscopic submucosal dissection: the different endoscopic techniques for colorectal tumors. J Surg Res. 2016 May;202(1):204–15. [ Links ]
22 Chen T, Qin WZ, Yao LQ, Zhong YS, Zhang YQ, Chen WF, et al. Long-term outcomes of endoscopic submucosal dissection for highgrade dysplasia and early-stage carcinoma in the colorectum. Cancer Commun (Lond). 2018 Mar;38(1):3. [ Links ]
23 Chiba H, Tachikawa J, Kurihara D, Ashikari K, Goto T, Takahashi A, et al. Safety and efficacy of simultaneous colorectal ESD for large synchronous colorectal lesions. Endosc Int Open. 2017 Jul;5(7):E595–602. [ Links ]
24 De Ceglie A, Hassan C, Mangiavillano B, Matsuda T, Saito Y, Ridola L, et al. Endoscopic mucosal resection and endoscopic submucosal dissection for colorectal lesions: A systematic review. Crit Rev Oncol Hematol. 2016 Aug;104:138–55. [ Links ]
25 Holmes I, Friedland S. Endoscopic Mucosal Resection versus Endoscopic Submucosal Dissection for Large Polyps: A Western Colonoscopist’s View. Clin Endosc. 2016 Sep;49(5):454–6.
26 Awadie H, Repici A, Bourke MJ. Endoscopic management of large nonpedunculated colorectal polyps: selective treatment algorithms are needed. Endoscopy. 2017 Mar;49(3):214–6. [ Links ]
27 Fuccio L, Repici A, Hassan C, Ponchon T, Bhandari P, Jover R, et al. Why attempt en bloc resection of non-pedunculated colorectal adenomas? A systematic review of the prevalence of superficial submucosal invasive cancer after endoscopic submucosal dissection. Gut. 2018 Aug;67(8):1464–74. [ Links ]
28 Shigita K, Oka S, Tanaka S, Sumimoto K, Hirano D, Tamaru Y, et al. Long-term outcomes after endoscopic submucosal dissection for superficial colorectal tumors. Gastrointest Endosc. 2017 Mar;85(3):546–53. [ Links ]
29 Kanesaka T, Toth E, Thorlacius H. Selection bias complicates comparisons of endoscopic submucosal dissection vs. endoscopic mucosal resection in the treatment of colorectal neoplasms. Endoscopy. 2017 May; 49(5): 511. [ Links ]
30 Barret M, Lepilliez V, Coumaros D, Chaussade S, Leblanc S, Ponchon T, et al.; Société Française d’Endoscopie Digestive (SFED). The expansion of endoscopic submucosal dissection in France: A prospective nationwide survey. United European Gastroenterol J. 2017 Feb;5(1):45–53.
31 Libânio D, Costa MN, Pimentel-Nunes P, Dinis-Ribeiro M. Risk factors for bleeding after gastric endoscopic submucosal dissection: a systematic review and meta-analysis. Gastrointest Endosc. 2016 Oct;84(4):572–86. [ Links ]
32 Kim SJ, Choi CW, Kang DH, Kim HW, Park SB. Second-look endoscopy and factors associated with delayed bleeding after endoscopic submucosal dissection. World J Gastrointest Endosc. 2016 Feb;8(3):173–9. [ Links ]
33 Goto O, Fujishiro M, Oda I, Kakushima N, Yamamoto Y, Tsuji Y, et al. A multicenter survey of the management after gastric endoscopic submucosal dissection related to postoperative bleeding. Dig Dis Sci. 2012 Feb;57(2):435–9. [ Links ]
Statement of Ethics
The authors have no ethical conflicts to disclose.
Disclosure Statement
The authors declare that there is no conflict of interest regarding the publication of this paper.
* Corresponding author.
Pedro Pimentel-Nunes
Department of Gastroenterology, Portuguese Oncology Institute of Porto
Rua Dr. Bernardino de Almeida
PT–4200-072 Porto (Portugal)
E-Mail pedronunesml@gmail.com
Received: March 28, 2019; Accepted after revision: May 11, 2019














