Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
GE-Portuguese Journal of Gastroenterology
Print version ISSN 2341-4545
GE Port J Gastroenterol vol.26 no.3 Lisboa June 2019
https://doi.org/10.1159/000490205
CLINICAL CASE STUDY
Carvedilol-Induced Liver Injury, a Rare Cause of Mixed Hepatitis: A Clinical Case
Hepatotoxicidade induzida por carvedilol, uma causa rara de hepatite de padrão misto – um caso clínico
João Ruaa, Ana Rita Pratab, Ricardo Marquesa, Rafael Silvaa, Bráulio Gomesa, João Fragac, Jorge Fortunaa
aServiço de Medicina Interna B, Centro Hospitalar e Universitário de Coimbra, Coimbra, Portugal; bServiço de Reumatologia, Centro Hospitalar e Universitário de Coimbra, Coimbra, Portugal; cServiço de Anatomia Patológica, Centro Hospitalar e Universitário de Coimbra, Coimbra, Portugal
* Corresponding author.
ABSTRACT
Introduction: Drug-induced liver injury is an increasingly prevalent consequence of the diversification of available therapeutic weapons, mostly idiosyncratic and with several possible mechanisms and patterns of specific damage for each drug. Carvedilol, a widely used non-selective alpha and beta blocker leads, in very rare cases, to injury of the bile ducts by toxic metabolites, resulting in a mixed-pattern hepatitis with possible progression to chronic cholestatic syndrome and cirrhosis. The authors report the second known case of this important toxicity. Clinical Case: An 83-year-old woman was admitted to the Internal Medicine ward for etiological clarification of a mixed-pattern hepatitis. Clinical history was unremarkable and structural, infectious, and autoimmune causes were excluded by blood tests and imaging exams, ultimately leading to the diagnosis of toxic hepatitis that was further confirmed by liver biopsy with morphologic findings of mixed-pattern liver injury. Carvedilol, started 6 months before, was deemed the causal agent since it was the only drug with a clinically, temporally, analytically, and histologically compatible pattern. The withdrawal of the drug resulted in slow reversal of the referred abnormalities. Conclusion: In very rare cases, carvedilol can cause important liver toxicity as a chronic cholestatic syndrome which can evolve to cirrhosis. It should be taken in consideration as causal agent in similar cases and stopped immediately upon suspicion, as the timely withdrawal results in reversion of the pathological findings.
Keywords: Carvedilol, Toxic hepatitis, Mixed-pattern hepatitis, Carvedilol-induced hepatotoxicity, Chronic cholestatic disease
RESUMO
Introdução: A lesão hepática induzida por drogas é uma consequência cada vez mais prevalente da diversificação de armas terapêuticas disponíveis. São principalmente reacções idiossincráticas, com vários mecanismos possíveis e padrões de danos específicos de cada droga. O carvedilol, bloqueador alfa e beta não seletivo amplamente utilizado, leva, em casos muito raros, a lesão dos canalículos biliares por metabólitos tóxicos, resultando numa hepatite padrão misto com possível progressão a síndrome colestática crónica e potencialmente cirrose. Os autores relatam o segundo caso conhecido desta importante toxicidade. Caso clínico: Uma mulher idosa foi admitida na enfermaria de Medicina Interna para esclarecimento etiológico de uma hepatite de padrão misto. Esta investigação que incluiu uma extensa pesquisa de antecedentes, exclusão de causas estruturais, infecciosas e auto-imunes por análises sanguíneas e exames de imagem, levou ao diagnóstico de uma hepatite tóxica, confirmada por biópsia hepática com achados morfológicos de um padrão misto de lesão hepática. O carvedilol, introduzido 6 meses antes, foi considerado o agente causal dado ser a única substância com padrão clínico, temporal, analítico e histológico compatível. A retirada do medicamento resultou na reversão lenta das referidas anormalidades. Discussão: Em casos muito raros, o carvedilol pode causar toxicidade hepática importante sob a forma de síndrome colestástica crónica que pode evoluir até uma cirrose hepática. Deve ser tomado em consideração como potencial agente causal em casos semelhantes e retirado imediatamente após suspeita, sendo que a suspensão atempada resulta na reversão completa dos achados patológicos.
Palavras-Chave: Carvedilol, Hepatite tóxica, Hepatoxicidade por carvedilol, Hepatite de padrão misto, Síndrome colestático crónico
Introduction
Drug-induced liver injury (DILI) is an increasingly prevalent consequence of the diversification of available therapeutic weapons. Hepatotoxicity is mostly idiosyncratic and can be caused by several possible mechanisms and manifest with different patterns of specific damage from each drug. Authorization to enter the market implies that these are rare events but their incidence varies. Carvedilol, a non-selective alpha and beta blocker used in heart failure, arterial hypertension, and also in variceal hemorrhage prophylaxis may, in very rare cases, lead to mixed hepatitis, secondary to injury of the bile ducts by toxic metabolites, with possible progression to chronic cholestatic syndrome and cirrhosis. To our knowledge there is only one published case reporting DILI associated with carvedilol.
Clinical Case
An institutionalized 83-year-old woman was referred to the Emergency Department due to severe hyperkalemia (7.1 mmol/L) detected in routine blood tests. Her only complaint was decrease of the urinary output in the previous days and pruritus for about 5 months. She denied abdominal pain, nausea, vomiting, or icterus. She was unaware of her usual medication and referred diabetes mellitus and chronic kidney disease (CKD) as her past medical history. On the physical examination, she was not severely ill, apyretic, and hemodynamically stable. Mild cutaneous mucosal dehydration, a panfocal systolic murmur, and moderate lower limb edema were the only abnormalities found. Abdominal examination was unremarkable, without hepatomegaly or jaundice, as was the rest of the physical exam. Severe hyperkalemia was confirmed by arterial blood sampling, which also showed a non-compensated mild metabolic acidemia (pH 7.27, HCO3 20.3 mmol/L, pCO2 44 mm Hg) without hyperlactacidemia but with hyperglycemia without associated ketonemia. The electrocardiogram did not present any relevant changes, and treatment of hyperglycemia and hyperkalemia was started. Complete blood count, clotting times, and complete biochemistry showed an exacerbation of chronic renal disease of probable pre-renal etiology: urea nitrogen 12.7 mmol/L (normal range [N] 1.3–3.5), creatinine 280 μmol/L (N 48– 90). A cholestatic pattern of liver injury was also noted (R value of 1.13; AST 67 U/L, ALT 110 U/L, alkaline phosphatase [ALP] 430 U/L, g-GT 936 U/L for N < 31, N < 34, N 40–150, and N < 38, respectively), and lactate dehydrogenase, total bilirubin, and C-reactive protein were within the normal range. Ultrasonography excluded biliary obstruction and showed a liver with regular contours and slightly heterogeneous texture with increased reflectivity probably due to steatosis, and also CKD-compatible kidneys without obstruction. After stabilization of the metabolic decompensation, she was hospitalized for etiological clarification.
The clinical background check allowed us to update the patient’s medical history: type 2 diabetes mellitus with retinopathy and nephropathy, stage 4 CKD, essential hypertension, mixed dyslipidemia, and hyperuricemia. A recent echocardiogram showed left ventricle type one diastolic dysfunction with normal ejection fraction, without other relevant abnormalities. There were no known episodes of acute heart failure. She was under isophane insulin 38 + 26 U and regular human insulin on demand, sitagliptin 25 mg once daily (od), folate and ferrous sulfate 1 + 90 mg od, losartan 50 mg od, carvedilol 6.25 mg twice daily (td), furosemide 40 mg td, venlafaxine 75 mg od, atorvastatin 20 mg od, allopurinol 100 mg od, diazepam 10 mg od, and hydroxyzine 25 mg td. She had been under spironolactone for a few weeks for unexplained reasons, previously withdrawn because of hyperkalemia. Household staff denied alcohol consumption, previous acute illness, or epidemiologic context such as illness in another user, trips to the countryside, contact with animals, or changes in eating habits. There was no history of previous drug reactions nor of other liver diseases. At the pharmacological level, in addition to the aforementioned spironolactone, only carvedilol (6 months) and hydroxyzine (5 months, for pruritus) had been started recently, with the remainder being used for several years. The patient had also undergone treatment for scabies because of chronic itching, without improvement.
Syphilis and viral causes such as hepatitis A, B, C, and E, cytomegalovirus, adenovirus, Epstein-Barr, and HIV were excluded. ANAs, ASMAs, AMAs, ENAs, and antibodies associated with hepatic autoimmunity (anti-LKM1, anti-SLA, and anti-LC1) were also absent. Ceruloplasmin and alpha-1 antitrypsin (α1AT) were not measured as Wilson and α1AT deficiency were considered very unlikely given the referred presentation. Erythrocyte sedimentation rate was 61 mm/h and ferritin was 431 ng/mL. Taking into account the data obtained and the absence of suggestion of infection, obstruction, and autoimmunity, toxic hepatitis and nonalcoholic steatohepatitis were considered the most probable hypotheses. Based on the LiverTox database, allopurinol, atorvastatin, carvedilol, sitagliptin, diazepam, and venlafaxine were identified as potentially hepatotoxic and suspended.
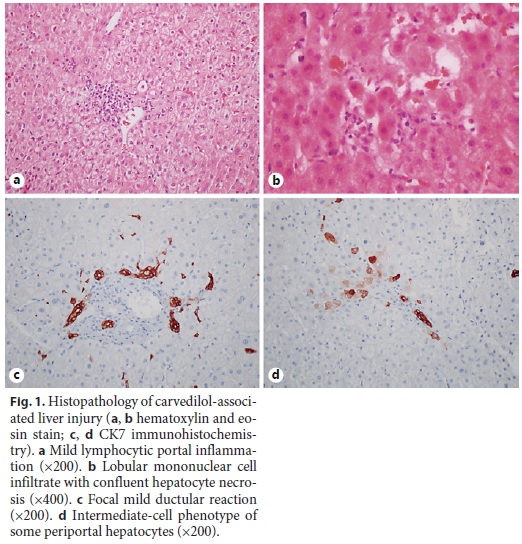
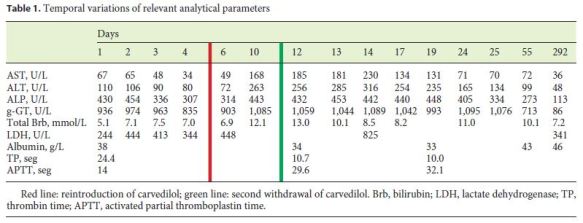
At the ward, the patient was clinically stable but the liver enzymes that initially improved with almost normalization of the transaminases, entered a new ascending phase from the 5th to the 13th day of hospitalization, to a maximum of 453, 1,098, 230, and 316 U/L for ALP, g-GT, AST, and ALT, respectively, with normal bilirubin (mixed pattern; R value 2.13). Retrospectively, it was verified that this aggravation coincided with the reintroduction of carvedilol, furosemide, losartan, and isophane insulin, besides initiation of enoxaparin at the time of the ward transfer. Due to the persistence of the alterations, an abdominal computed tomography scan was performed and showed normal liver with regular contour and homogeneous texture, excluding steatosis, gallbladder, biliary tract and pancreatic changes, abdominal adenopathy, and peritoneal effusion. Given the diagnostic uncertainty, a liver biopsy was performed and the histological examination revealed normal portal tracts with mild inflammatory infiltrate of mononuclear cells without interface damage and with mild periportal ductular reaction highlighted with CK7 (Fig. 1). There were no copper deposits in the periportal hepatocytes but some had intermediate-cell phenotype (cytokeratin 7 expression). The hepatocytes had discrete anisocytosis, anisocariosis, and some mitoses. There were some focal acinar inflammatory foci with hepatocyte necrosis but no steatosis was observed and iron deposits in Kupffer cells were discrete. These findings suggest a mixed pattern of liver injury with hepatocellular inflammation and necrosis and mild signs of chronic cholestasis, consistent with the histological pattern present in the only reported case associated with carvedilol [1, 2]. The patient had already been discharged when the result was made available, in clinical stability and with improving liver tests and maintenance of the suspension of carvedilol (withdrawn again since the above-mentioned recrudescence), considered the most likely etiological agent. She was reassessed in an outpatient visit 1 month after the discharge, in clinical stability and with the liver tests tending to normalization (AST 72 U/L, ALT 99 U/L, ALP 99 U/L, and g-GT 213 U/L), and 9 months later, already with liver function tests within normal ranges (Table 1).
Discussion
DILI is the most frequent adverse drug effect, ranging from discrete and asymptomatic enzymatic changes to acute hepatic failure [3, 4]. More than 900 drugs, herbs, and toxins have documented this possibility, being the most frequent cause of acute hepatic failure in developed countries and the biggest responsible factor for drug withdrawal from the market [5–7]. In most cases, the incidence of DILI is extremely low, varying between 1/10,000 and 1/100,000 [8]. They are usually idiosyncratic reactions that occur in susceptible patients, without a clear dose-response relation, being associated with certain HLA alleles [3, 4]. Clinical and laboratory manifestations are varied in latency, presentation, and course. Most drugs are lipophilic, facilitating intestinal absorption, being then transported to the liver where they are hydrophilized through metabolization primarily by cytochrome P450 and conjugation with glucuronides, sulfates, or glutathione, and subsequently excreted into the systemic circulation [6]. In this intrahepatic stage, toxicity can occur by several mechanisms whose changes caused in intracellular organelles determine a pattern of liver injury. These alterations result from several mechanisms such as the alteration of intracellular proteins through covalent bonds generated through cytochrome P450, disruption of bilirubin transport proteins in the canalicular membrane, formation of adducts (drug-enzyme bonds) with immunogenic potential that lead to cell destruction mediated by antibodies or due to direct cytotoxic action, activation of apoptosis through TNF-alpha and Fas pathways, direct injury of the mitochondria with compromise of aerobic respiration and oxidative stress, and direct lesion of the bile canaliculi by toxic metabolites excreted in bile [6].
Carvedilol is a non-selective alpha and beta blocker, used in the treatment of heart failure, arterial hypertension, and variceal hemorrhage prophylaxis. It is a lipophilic substance undergoing extensive metabolization in its first hepatic passage, with predominantly biliary excretion in the form of inactive metabolites, following glucuronization [9]. Although 2% of patients report a slight elevation of transaminases, relevant hepatotoxicity is very rare, with only 1 previously described case [1, 2]. DILI associated with carvedilol was reported to be an idiosyncratic reaction with a latency period of 5 to 90 days and a lesion pattern characteristic of chronic cholestasis [6]. Due to its rarity, the exact mechanism responsible for hepatotoxicity is unclear, although the mixed pattern of enzymatic elevation without bilirubin elevation observed in both cases appears to indicate direct damage of the bile ducts by metabolites of carvedilol excreted through the bile duct and secondary hepatocyte injury [2, 6, 10–12]. This phenomenon is described as cholangio-destructive cholestasis, and, in the absence of suspension of the causative agent, may lead to chronic cholestatic damage similar to primary sclerosing cholangitis [10, 12].
The diagnosis of drug-induced hepatotoxicity is typically difficult as several patterns of hepatic enzyme and histological changes with variable latency are possible, being capable of mimicking practically all liver diseases and being largely a diagnosis of exclusion. The key to diagnosis is an adequate investigation focused on the temporal evolution of changes with concordant sequence and latency period, correct exclusion of other drugs, previous liver disease, interactions and complications of other underlying diseases, identifying a pattern of analytical changes and histological results expected for the causative agent and, finally, improvement after discontinuation [3, 10, 13]. Some complex scores (e.g., Roussel-Uclaf Causality Assessment Method, RUCAM) have been developed in order to aid in DILI diagnosis, although expert clinical judgement and a thorough exclusion of other causes of liver injury are generally recommended in clinical practice, with the aid of literature review (LiverTox).
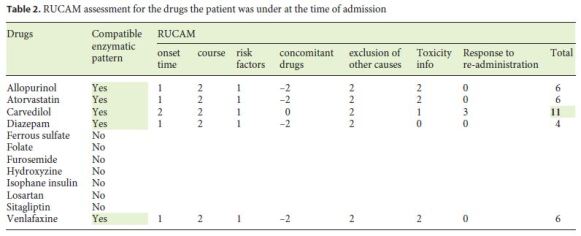
In this case, the patient was started on carvedilol 6 months earlier, developing non-specific pruritus 1 month later, aggravated by an increase in dosage. To this suggestive temporal pattern is added a clinical, enzymatic, and histological pattern consistent with the only reported case of severe carvedilol toxicity, a mixed-pattern hepatitis without hyperbilirubinemia, severe pruritus, and histological changes consistent with mixed-pattern liver injury and mild chronic cholestatic syndrome [1]. The biopsy, usually not mandatory, was of particular importance due to the rarity of carvedilol DILI. It showed abnormalities compatible with the supposed mechanism of injury and its chronicity, also allowing us to exclude liver deposit-associated pathologies and also autoimmune, viral, and non-alcoholic steatohepatitis due to absence of interface hepatitis. We highlight the slow and progressive improvement of the enzymatic abnormalities after drug discontinuation and immediate recrudescence after drug re-introduction, which was interpreted as an unintentional but objectively positive provocation test [7]. Other of her previous medicines were reintroduced but none had known liver toxicity. The RUCAM was calculated (Table 2), obtaining a result of 11, classifying this reaction as highly probable [14]. In view of the above, the authors believe that the causal relationship between the described alterations and carvedilol use is proven, and this case intends to alert to this very rare but possible and serious toxicity, which should be taken into account when the clinical and analytical framework fits and motivates the immediate suspension of the drug.
Finally, and despite the valuable contribution of our rechallenge to achieving a definite diagnosis, it is important to state that it is strongly discouraged due to the potential of severe life-threatening anamnestic response and acute liver failure, being associated with a 15% mortality. It should only be considered for critical medicines without a valid alternative. Polymedication can pose difficulties, often requiring suspension of several agents when a causal drug is not identified.
References
1 Hagmeyer KO, Stein J: Hepatotoxicity associated with carvedilol. Ann Pharmacother 2001;35:1364–1366. [ Links ]
2 National Library of Medicine: LiverTox: Carvedilol. https://livertox.nlm.nih.gov//Carvedilol.htm. [ Links ]
3 Habib S, Shaikh OS: Drug-induced acute liver failure. Clin Liver Dis 2017;21:151–162. [ Links ]
4 Kataray D, Verma S: Drug-induced liver injury. Clin Med 2016;16:s104–s109. [ Links ]
5 Medscape: Mehta N: Drug-Induced Hepatotoxicity. https://emedicine.medscape.com/article/169814-overview . [ Links ]
6 Lee WM: Drug-induced hepatotoxicity. N Engl J Med 2003;349:474–485. [ Links ]
7 Alempijevic T, Zec S, Milosavljevic T: Druginduced liver injury: Do we know everything? World J Hepatol 2017;9:491–532. [ Links ]
8 Luo G, Shen Y, Yang L, et al: A review of druginduced liver injury databases. Arch Toxicol 2017;91:3039–3049. [ Links ]
9 Morgan T: Clinical pharmacokinetics and pharmacodynamics of carvedilol. Clin. Pharmacokinet 1994;26:335–346. [ Links ]
10 Kleiner D: Drug induced liver injury – the hepatic pathologist’s approach. Gastroenterol Clin N Am 2017;46:273–296.
11 Jaeschke H, Gores G, Cederbaum A, et al: Mechanisms of hepatotoxicity. Toxicol Sci 2002;65:166–176. [ Links ]
12 Madame Curie Bioscience database: KullakUblick GA: Drug-induced cholestatic liver disease. https://www.ncbi.nlm.nih.gov/books/NBK6102/. [ Links ]
13 Goodman ZD: Phenotypes and pathology of drug-induced liver disease. Clin Liver Dis 2017;21:89–101. [ Links ]
14 Danan G, Benichou C: Causality assessment of adverse reactions to drugs-I. A novel method based on the conclusions of International Consensus Meeting: application to drug-induced liver injuries. J Clin Epidemiol 1993;46:1323–1330. [ Links ]
Statement of Ethics
This study did not require informed consent nor review/approval by the appropriate ethics committee.
Disclosure Statement
The authors have no conflicts of interest to declare.
* Corresponding author.
Dr. João Miguel Ferreira Rua
Serviço de Medicina Interna B, Centro Hospitalar e Universitário de Coimbra
Alameda Dr. Armando Gonsalves nº 15 ap 301
PT–3000-059 Coimbra (Portugal)
E-Mail joaomfrua@gmail.com
Received: March 13, 2018; Accepted after revision: May 11, 2018














