Introduction
The Pediatric Early Warning Systems (PEWS) were developed to maximize early recognition of clinical deterioration in children by identifying hospitalized patients who were likely to require resuscitation to treat cardiopulmonary arrest.1-3 Multiple studies showed that an elevated PEWS is associated with need for pediatric intensive care units (PICU) admission from the emergency department (ED) or pediatric wards.4-10 Multiple PEWS have been implemented around the world with some evidence revealing improved outcomes in clinical deterioration recognition.11-15
A study from 2017 suggested PEWS to be effective on predicting deterioration in different subspecialty acute care patients.16 It has been applied in other contexts with good results, such as in the oncology field,17-21 burn centres,22 and in the peritransport setting.23
A review from 2019 suggested that the use of PEWS in resource-limited settings has potential to reduce mortality while also reducing resource utilization.24
Despite the multiple uses of this score, the specific application in the context of SSU is not well established.
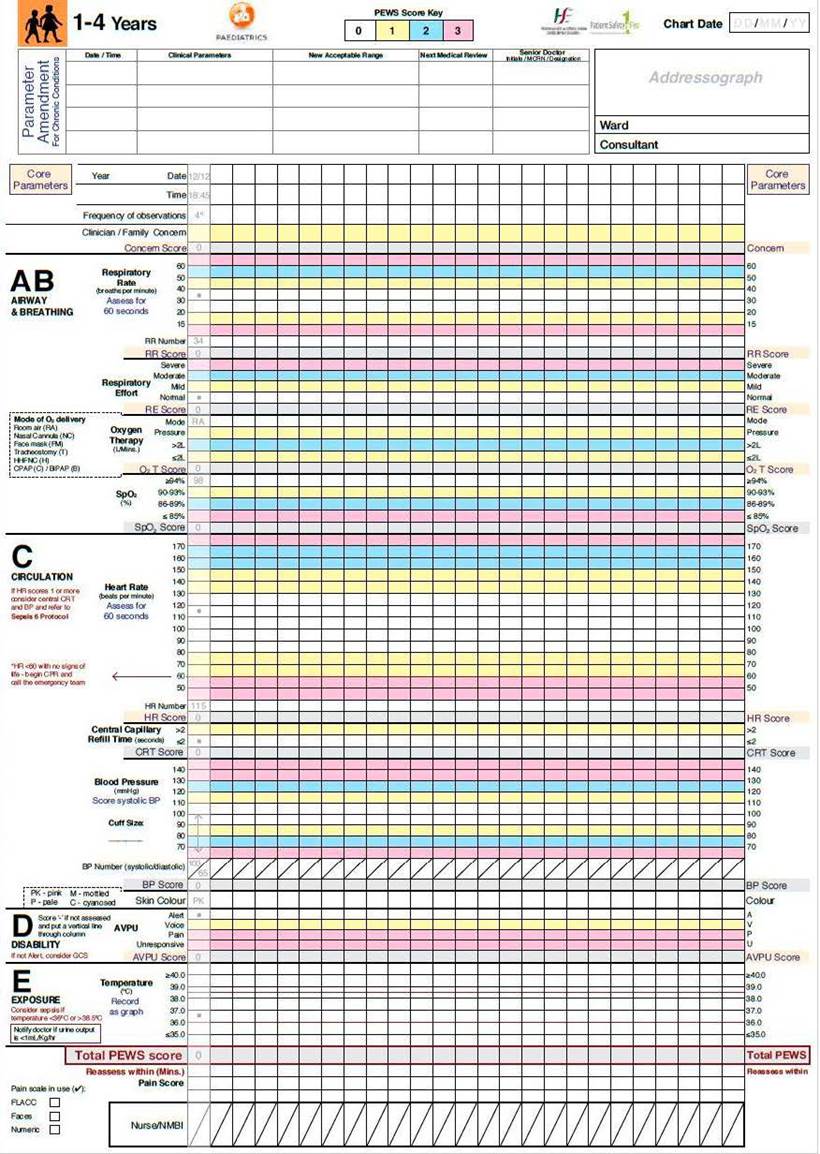
Among the PEWS available in the literature, our hospital selected the Irish PEWS because it considers vital signs by age group.25 It was implemented in July 2017 in our pediatric SSU, aiming to improve clinical monitoring in our unit. This version of the PEWS scale was translated at our center.
This study evaluates whether the implementation of the PEWS score in our SSU was effective on improving early identification of patients with clinical deterioration, by analyzing its sensitivity and specificity globally in our population. We also aimed to look for performance variations in different age and nosologic groups.
Methods
Setting
The study setting was the SSU of a private urban general hospital located in Lisbon, Portugal, with an average of 41563 pediatric visits per year, with a rate of SSU admission of 1.50%. No specific monitoring protocol was in place before this study.
Intervention design
The Irish PEWS has specific charts for 0-3 months-old, 4-11 months-old, 1-4 years-old, 5-11 years-old and ≥12 years-old. It is a 5-component system, including core parameters, airway and breathing, circulation, disability and exposure. The score varies between 0 and 22. We translated charts (Fig. 1) and user manual to Portuguese and provided training to the pediatric ED team, involving nurses and physicians. PEWS scoring occurred at the admission to the SSU. Then, according to the score, a minimum observation, a minimum alert, and a minimum response were defined.26 We defined clinical deterioration as the need for PICU transfer or high flow oxygen (HFO) therapy (or higher level of respiratory support).
Intervention implementation and monitoring
Educational resources were used for motivating and training staff, including instructions on how to use the charts, training modules, and case studies. We monitored implementation fidelity through audits based on rates of charts’ utilization, completion and accuracy of PEWS scoring.
Data collection
We collected data using three methods: by reviewing electronic medical records, by reviewing manual PEWS charts registries, and by applying online surveys to providers.
We retrospectively reviewed medical records of patients admitted do the SSU during the 32-months study period, from July 2017 to February 2020. The inclusion criteria were patients who (i) were admitted to the SSU during the study period after PEWS implementation, and (ii) stayed in SSU for more than 1 h, and (iii) had at least one PEWS score applied during the stay. We stratified our sample to include patients across all age groups (0-3 months, 4-11 months, 1-4 years, 5-11 years, and 12-17 years). We extracted data from the medical records, which was introduced in an IBM SPSS® dataset sheet, having been applied the security and privacy measures in accordance with the Hospital Ethics Committee and Data Protection Officer policy.
Data analysis
Statistical analysis was carried out using IBM SPSS version 23.0® software. We used descriptive statistics to calculate the overall characteristics of patient medical records and to analyse responses to the online survey. Values were expressed as median and interquartile range (IQR) for quantitative variables and percentages for qualitative variables. For the comparative study, data was grouped in classes. To compare quantitative variables between subgroups we used the Mann-Whitney U test. We used receiver operating characteristic (ROC) curves to evaluate test performance by testing the relationship between PEWS scores and clinical deterioration, both for the entire sample, and for some subgroups. Based on the area under the curve (AUC) obtained, we defined the test accuracy as excellent (0.900-1.000), good (0.800-0.900), fair (0.700-0.800), poor (0.600-0.700), and fail (0.500-0.600).26 The level of significance considered was p<0.05.
Results
Collected data
We included 1323 pediatric patient records in the study. The median age was 3.07 years-old with a minimum of 5 days-old and a maximum of 17 years and 361 days old. Patient distribution by age group was the following: 15.6% (n=206) from 0 to 3 months; 14.4% (n=191) from 4 to 11 months; 24.9% (n=329) from 1 to 4 years; 26.7% (n=353) from 5 to 11 years; and 18.4% (n=244) from 12 to 17 years.
Patient characterization
The most frequent diagnosis for admission to SSU was gastrointestinal pathology, accounting for 34.3% (n=454) of total admissions, followed by respiratory (29.7%, n=393) and neurologic (9.8%, n=129) diseases. Fig. 2 displays a more detailed nosological classification. Gastrointestinal disorders were mostly characterized by vomiting, diarrhea and/or unspecific abdominal pain. Bronchiolitis was responsible for 49.6% (n=195) of admissions for respiratory pathology, followed by wheezing and pneumonia, accounting for 14.0% (n=55) and 5.6% (n=22), respectively. Regarding neurologic diseases, head trauma was the most common diagnosis (27.1%, n=35), followed by seizures (25.6%, n=33).
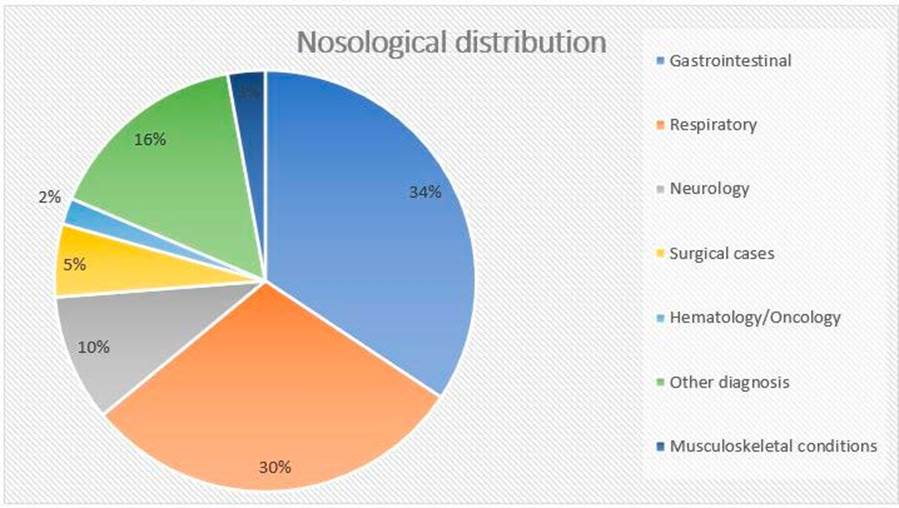
Figure 2 Patients’ distribution by diagnosis. Among the other diagnosis group: Skin and soft tissues (n=25, 1.9%); Acute intoxication (n=23, 1.7%); Nephrology (n=22, 1.7%); Endocrinology (n=19, 1.4%); Cardiology (n=10, 0.8%); Anaphylaxis (n=10, 0.8%); Sepsis (n=7, 0.5%); Others (n=92, 7.0%).
After discharge from SSU, 54.8% (n=725) of patients were sent home, 40.7% (n=538) were transferred to our pediatric ward, 2.6% (n=35) were transferred to public residency area hospital, and 1.9% (n=25) required a higher level of care, being transferred to PICU. Fifty-five patients (4.2%) needed HFO therapy. Using clinical criteria, physicians considered that 68 (5.7%) had clinical deterioration.
PEWS score results
The median of PEWS score among all studied medical records was 2.00 (IQR 1.00-3.00). The maximum value was equal or greater than 3 in 13.4% (n=177) patients, equal or greater than 4 in 8.5% (n=112), and equal or greater than 5 in 5.4% (n=71). Approximately two thirds (65.2%, n=284) had a maximum PEWS score of 2 or lower.
Dividing by nosological groups, the highest maximum PEWS score medians were in the context of respiratory pathology, sepsis, acute intoxication, and cardiovascular diseases, with values of, 4.00 (IQR 2.00-5.00), 2.00 (IQR 2.00-7.00), 2.00 (IQR 1.00-4.00), and 2.00 (IQR 1.75-3.00), respectively. In the opposite way, gastrointestinal and musculoskeletal conditions had the lowest scores, both with median values of 1.00 (IQR 0.00-2.00). Particularly for neurologic disease, a significant PEWS score median difference was found between the seizure group (3.00, IQR 1.00-4.00) and the non-seizure group (1.00, IQR 1.00-2.00) (p<0.001).
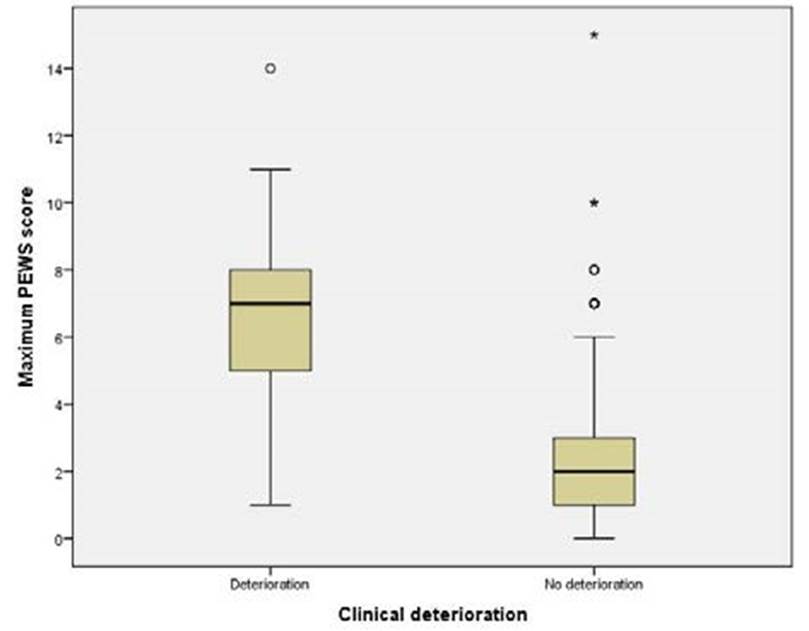
Among patients who were considered to have clinical deterioration, the maximum PEWS score median was 7.00 (IQR 5.00-8.00), while for those who were not, the median was 2.00 (IQR 1.00-3.00). Maximum PEWS score variations between the clinical deterioration and the non-clinical deterioration groups are represented by Fig. 3 (p<0.001).
Test performance
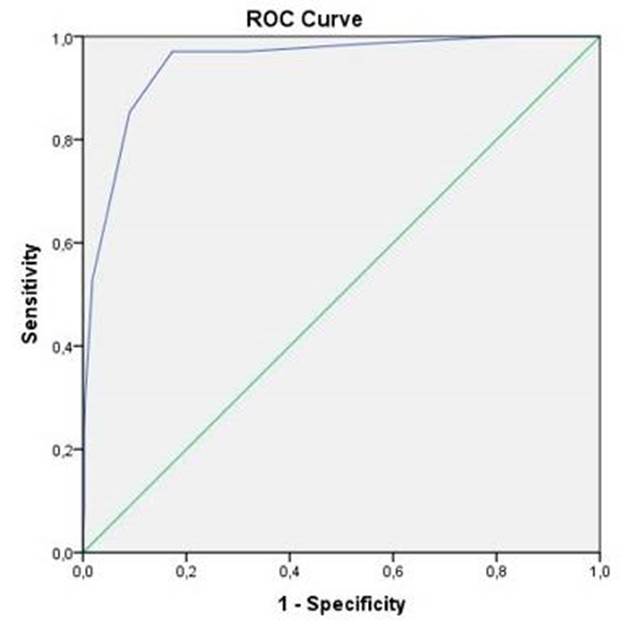
A ROC curve was used to analyse the predictive value of PEWS score for clinical deterioration in our sample. The AUC was 0.947 and the best cut-off value was 4, obtaining a sensitivity 97.1% of and a specificity of 82.7%. Using a score of 3 as the cut-off value, a sensitivity of 97.1% and a specificity of 68.6% were achieved. Using a score of 5 as the cut-off value, sensitivity falls to 85.3% but specificity increases to 91.0%. The ROC curve is represented in Fig. 4.
Additionally, ROC curves were designed for each age and nosological groups. Between age groups, the AUC ranged from a minimum of 0.901 for the group of 1 to 4 years old children and a maximum of 0.977 for the group including 12 to 17 years old adolescents.
Within the respiratory pathology group, despite an AUC of 1.000 calculated for pneumonia, only one patient with this diagnosis was considered to have clinical deterioration. For bronchiolitis, using 5 as the cut-off value, PEWS score was able to reach a sensitivity of 88.9% and a specificity of 73.3%.
In the neurologic group, the non-seizure subgroup ROC curve had an AUC of 0.902, and a cut-off value of 2 proved to have a sensitivity of 100% and a specificity of 56.5%. In the seizure subgroup, despite some high PEWS scores, none of the 33 patients were considered to have clinical deterioration, and so a ROC curve was not performed.
The AUC calculated for the sepsis group was 0.500. The sample of patients with sepsis included 7 children. Three (42.9%) needed transfer to PICU. Of those, 2 had a maximum PEWS score greater than 4; the other child had a maximum PEWS score of 1, having been in our SSU for 4 hours until the transfer, and leaving with a diagnosis of sepsis with suspected disseminated intravascular coagulation, without cardiac, respiratory or neurological compromise. The remaining 4 patients (57.1%) who did not have clinical deterioration were diagnosed with clinical sepsis; 3 of which were newborns with maximum PEWS score equal to or less than 2; the other patient had a maximum PEWS of 8, recorded when he was febrile, being all other records equal to or less than 3. (Fig. 5)
It is important to emphasize that despite the high AUC values for musculoskeletal, cardiology, and hematology/oncology diseases, each of these groups had only one patient considered to have clinical deterioration.
The AUC calculated for each nosological group and for each diagnosis within the respiratory group is in Table 1.
For nephrology, gastrointestinal, surgical, skin and soft tissues, endocrinology, anaphylaxis and other groups, estimation could not be performed for lack of statistical power.
Table 1 AUC calculated by nosological group and by respiratory diagnosis.
| Nosological group | Area | Asymptotic 95% Confidence Interval | |
| Lower Bound | Upper Bound | ||
| Neurology | 0.849 | 0.700 | 0.998 |
| Respiratory Bronchiolitis Recurrent wheezing/asthma Pneumonia Others | 0.905 0.896 0.829 1.000 0.947 | 0.869 0.849 0.658 1.000 0.892 | 0.940 0.944 0.999 1.000 1.000 |
| Musculoskeletal conditions | 1.000 | 1.000 | 1.000 |
| Cardiology | 1.000 | 1.000 | 1.000 |
| Hematology/Oncology | 0.981 | 0.924 | 1.000 |
| Sepsis | 0.500 | 0.000 | 1.000 |
| Acute intoxication | 0.909 | 0.779 | 1.000 |
Discussion
The PEWS score implementation in the context of our SSU revealed to be valuable for clinical practice, proving to be an excellent tool for early identification of clinical deterioration in our population. The different age groups studied did not significantly influence the accuracy of the test. Regarding the PEWS score performance in the different nosological groups, some differences were found. In some nosological groups, such as neurological, respiratory, musculoskeletal, cardiology, hematology/oncology and acute intoxications, the test performed particularly well, with high AUC values and identification of cut-off values with good sensitivities and specificities. These results are in line with previous studies carried out in the field of oncology, in which PEWS implementation proved to aid in triage between intermediate care units and PICU,17,18 and to be cost effective, resulting in a reduction in the number of unplanned PICU transfers.(19-21 The bedside PEWS also proved to be an accurate screening tool to predict clinical deterioration in stem cell transplant patients.22,23
Specifically, in the groups of respiratory and cardiovascular pathology, median values of maximum PEWS score were higher than for other nosological groups. This finding may be justified by the fact that both respiratory and cardiovascular diseases affect the organs that mostly influence vital signs, the variables that weight the most in the PEWS score calculation.
Despite a recent study stating PEWS as an independent risk factor for the death of children with sepsis, the ROC curve designed in our study for patients diagnosed with sepsis estimated an AUC with an unsatisfactory value, and so we considered important to interpret these results. Our sample of patients with sepsis is small. Importantly, the child who was classified with clinical deterioration and had a maximum PEWS value of 1, stayed in PICU for only a few hours, and did not need respiratory or hemodynamic support. It was not a real clinical deterioration. Another child with a maximum PEWS of 8 who did not deteriorate, had the highest score recorded when he was febrile, being all the other scores below 4.
We should interpret with caution scores of patients with seizures. These often reach high values, without meaning a subsequent clinical deterioration. Most likely, the reason is that those values were registered during the seizure or in the postictal period.
A major limitation of our study is that we used a self-translated scale not formally validated for Portuguese. This could be an important issue to develop soon. Another limitation is that we only considered the maximum PEWS score in the data collection and not the evolution of the scores during the observation period, which could give us a longitudinal view of the patients’ clinical evolution. In some nosologic groups, the number of patients who deteriorated clinically was low, which makes it difficult to interpret the test performance in these subgroups.
Conclusion
This study reinforces that early identification of children at risk of clinical deterioration is important and the use of PEWS may help in recognizing the early signs of serious illness. This score performed particularly well in the respiratory and neurologic groups. In the opposite direction, it did not prove to be a good tool to predict deterioration in children with sepsis.