Introduction
The myeloproliferative neoplasms (MPN) are clonal myeloid malignancies of the bone marrow with unique genetic etiology and hematological histomorphologic features. The broadest classification is by BCR-ABL (Philadelphia chromosome) status (Heppner et al., 2019). Polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF), the classical and most common BCR-ABL negative (Ph neg) MPN, are characterized by abnormal production of one or more myeloid lineages (Arber et al., 2016; Ana Paula Azevedo et al., 2017; Duncombe et al., 2020), and mostly due to somatic driver mutations in Janus kinase 2 gene, which are present in more than 95% of patients with PV and more than 50% of patients with MF and ET (Arber et al., 2016; Ana Paula Azevedo et al., 2017; McMullin & Anderson, 2020; Ayalew Tefferi, 2012; Ayalew Tefferi & Vainchenker, 2011).
Epidemiological estimates vary worldwide (Ana Paula Azevedo et al., 2017; Byun et al., 2017; Deadmond & Smith-Gagen, 2015; Duncombe et al., 2020; Heppner et al., 2019; Moulard et al., 2014; Roaldsnes et al., 2017; Titmarsh et al., 2014), with an MPN estimated annual incidence rate of 2.17 cases per 100,000 of the population and pooled annual incidence rates for PV, ET, and PMF of 0.84, 1.03, and 0.47 per 100,000, respectively (McMullin & Anderson, 2020; Titmarsh et al., 2014). Although MPN are considered rare disorders relative to solid tumors, they are an increasingly prevalent global healthcare issue, inducing substantial economic and social burdens (Byun et al., 2017; Petruk & Mathias, 2020).
During the course of disease and heterogeneous evolution, major cardiovascular (CV) events and severe bleeding, or disease progression can occur, directly affecting the morbidity and survival of patients (McMullin & Anderson, 2020; Petruk & Mathias, 2020; Seguro et al., 2020; Shallis et al., 2020). In fact, PV and ET can transform to myelofibrosis (secondary or post-ET/post-PV MF), acute myeloid leukemia, and, less commonly, myelodysplasia (Koschmieder, 2020; Kucine, 2020; Petruk & Mathias, 2020; Seguro et al., 2020; Vannucchi & Guglielmelli, 2017). Treatment aims at preventing these complications through various cytoreductive agents and disease modulation. Allogeneic stem cell transplantation is a potentially curative therapy but associates with significant morbidity and mortality and is thus reserved for patients with a poor prognosis (Koschmieder, 2020; Petruk & Mathias, 2020).
Prognosis and symptom presentation depend on the MPN subtype, symptom profiles varying greatly from asymptomatic to burdensome (Anderson et al., 2015; Brochmann et al., 2017; Emanuel et al., 2012; Geyer et al., 2014; Johansson et al., 2012; R. Mesa, Miller, et al., 2016; R. A. Mesa et al., 2007; R. Scherber et al., 2011; R. M. Scherber et al., 2016; Ayalew Tefferi et al., 2014). Common symptoms include fatigue; splenomegaly, with associated abdominal pain, discomfort and early satiety; constitutional symptoms (fever, night sweats, and weight loss); and also aquagenic pruritus, inactivity, concentration problems, and bone pain (Abruzzese et al., 2018; Emanuel et al., 2012; Moulard et al., 2014; Petruk & Mathias, 2020; R. Scherber et al., 2011; Vannucchi & Guglielmelli, 2017).
Numerous studies have shown that MPN result in marked impairment of patients’ quality of life (QoL), and associate with significant emotional issues, including depression and anxiety (Brochmann et al., 2019; Geyer et al., 2017; Harrison et al., 2017; McFarland et al., 2018; R. Mesa et al., 2018; Petruk & Mathias, 2020; Snyder & Chang, 2019; Yu et al., 2018, 2019). Thus, addressing disease burden in patients with MPN as part of the overall management strategy has become crucial to minimize impact on their daily lives, emotional and physical well-being (Petruk & Mathias, 2020). The assessment and continuous monitoring of symptom burden during the course of treatment using the MPN Symptom Assessment Form Total Symptom Score (MPN-SAF TSS-10 ITEMS or MPN-10) (R. Scherber et al., 2011), allows the detection of changes in symptomology that may be signs of disease progression and, therefore, can be used as an indicator of the need to reassess the disease evolution and/or therapeutic approach (R. Mesa, Mayo Clinic Cancer Center, et al., 2016). The systematic application of the MPN-10 scale has proved to be effective in reducing patients’ symptomatic burden and improving their QoL; however, there is no systematic collection of these data that allows the characterization of patients’ symptoms and clinical progress in real clinical practice.
Objective
This article aims to present the preliminary results of a prospective registry of Portuguese MPN patients followed at a multidisciplinary setting, including patients’ demographics and clinical characterization, and disease symptomatic burden through the MPN-10 scale assessment.
Study design
National, multicenter, prospective registry of MPN patients followed at nursing consultations, conducted from September 2018 to the end of June 2019, in 6 Portuguese sites: Centro Hospitalar de Lisboa Norte, CHLN (n=13); Centro Hospitalar de Lisboa Ocidental, CHLO (n=67); Centro Hospitalar de Trás-os-Montes e Alto Douro, CHTMAD (n=26); Centro Hospitalar de Vila Nova de Gaia/ Espinho, CHVNG (63); Centro Hospitalar do Baixo Vouga, CHBV - Aveiro (n=14); ULS de Matosinhos (n=141).
Study population
Patients who fulfilled the inclusion criteria (age ≥ 18 years old; MPN diagnosis of ET, PV or MF; written informed consent) were invited to participate in the study, described the study’s purpose and content and emphasized that their participation was voluntary. The patients were not offered compensation for participation.
Questionnaires and additional questions
MPN-10, a symptom load self-assessment validated tool, was used in this study. MPN-10 lists ten symptoms: fatigue; early satiety; abdominal discomfort; inactivity, problems with concentration, night sweats, itching (pruritus), bone pain (diffuse not joint pain or arthritis), fever (>37.8°C), and unintentional weight loss in the last 6 months, and the score ranges from 0 to 100 (R. Scherber et al., 2011). MPN-10 is a disease-specific questionnaire that specifically investigates the symptoms and QoL in MPN patients, recommended in the National Comprehensive Cancer Network (NCCN) Guidelines® to assess symptom burden at baseline and symptom status monitoring during the course of MPN treatment (R. Mesa, Mayo Clinic Cancer Center, et al., 2016). This questionnaire was translated into the Portuguese language and validated before this study (Sánchez et al., 2019). To standardize the application of the MPN-10 self-completion scale, the guidelines of the support guide for the symptoms’ assessment in patients with myeloproliferative syndromes using the MPN-10 scale were considered (Rocha et al., 2018).
Data collection and study assessments
At the initial assessment, data collection took place in a face-to-face visit, while follow-up visits (FU) could be performed over the phone. Information regarding age, sex, working situation, MPN subtype according to WHO diagnostic criteria (Arber et al., 2016), date of diagnosis, International Prognostic Scoring System (IPSS) (Cervantes et al., 2009) at diagnosis and Dynamic International Prognostic Scoring System (DIPSS) (Passamonti et al., 2010) risk score, therapy (support therapy and current treatment), clinical parameters (hemoglobin concentration, Hb; hematocrit, HTC; and platelet counts, PLT), and symptomatic burden based on MPN-10 were collected. The disease duration at the study entry was established using the MPN diagnosis date included in the patient clinical file. The distributions of patients on MPN subtypes were calculated.
Statistical analysis and missing data
Descriptive statistics of the variables collected in the initial evaluation (baseline) were used to present patients’ characteristics. The assessment of differences/ changes between baseline and each FU visit was performed trough paired t-test for continuous variables if the assumptions verified, or as alternative, Mann-Whitney non parametric test. Chi-square test was used to test the independence of baseline characteristics of categorical variables amongst MPN subtypes (ET, PV, MF). Missing data were not considered in the statistical analysis, which was performed using software SPSS, version 24.0. A p<0.05 was considered statistically significant.
Ethics
This study was conducted in full conformance with the ICH E6 Guideline for Good Clinical Practice and the Declaration of Helsinki principles. It was approved by the institutional review boards or ethics committees of the study sites. Patients’ agreement of consent was attained before the inclusion in the study. During the study, the participants had the opportunity to contact the study team in person, by phone and email, in order to clarify any questions regarding the study, their participation, or the MPN-10 issues.
Results
Patients
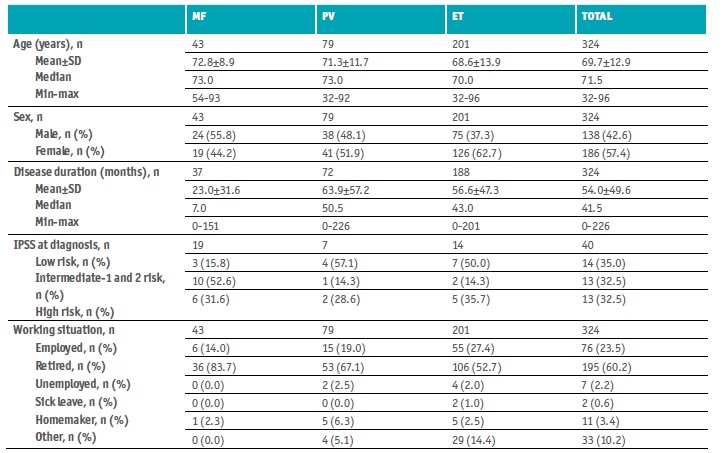
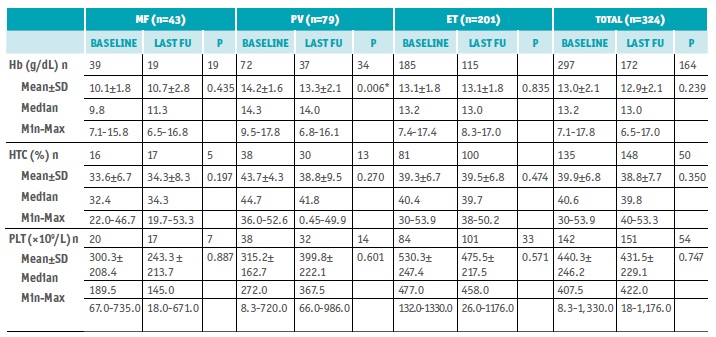
Of the 324 patients enrolled, 138 were male (42.6%) and 186 females (57.4%), the male to female ratio was 0.7:1 (Table 1). Most patients were retired (n=195, 60.2%) while 23.5% were still active (n=76). The age ranged from 32 to 96 years old, median age 71.5 years, and median disease duration > 3 years (41.5 months). At baseline, the median Hb level was 13.2 g/dL (range, 7.1-17.8 g/dL), the median HTC was 40.6% (0.3-53.9%), and the median PLT was 407.5 × 109/L (8.3-1,330.0 × 109/L).
Most patients had ET (n=201, 62.2%), 79 had PV (24.5%), and 43 MF (13.3%; 73.8% PMF and, if secondary MF, 72.7% post-ET).
IPSS evaluation at diagnosis found 14 low-risk patients, 13 intermediate-1 and 2 risk patients, and 13 high-risk patients. One hundred and thirty-three patients were older than 65 years; 10 had constitutional symptoms; 11 had a Hb lower than 10 g/dL; leukocyte count higher than 25x109/L was found in 2 patients; circulating blasts ≥ 1% in 4 patients; PLT lower than 100x109/L in 2 patients; 6 patients needed red cell transfusion; and 3 had unfavorable karyotype. The mean (±SD) DIPSS risk score was 1.1±0.6 (n=237), with 4 low-risk patients (1.7%), 225 intermediate-1 and 2 risk patients (95%), and 8 high-risk patients (3.3%).
Table 3 Baseline therapy
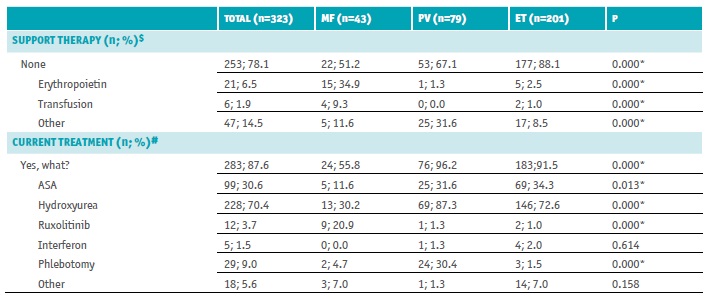
ET - essential thrombocythemia; MF - myelofibrosis; n - number of patients; PV - polycythemia vera
$ Multiple choice: none; erythropoietin; transfusion; other (specify)
# Multiple choice: ASA - acetylsalicylic acid; hydroxyurea; ruxolitinib; interferon; phlebotomy; other
* Statistically significant differences - χ2 test
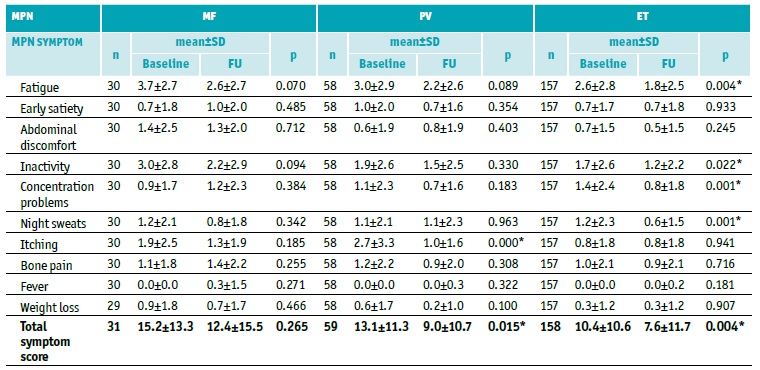
Table 4 MPN-10 items score per MPN subtype at baseline
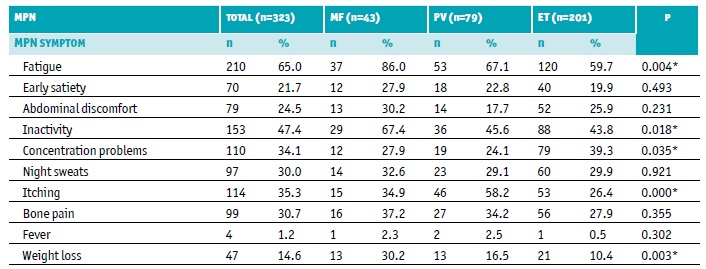
ET - essential thrombocythemia; MF - myelofibrosis; MPN - myeloproliferative neoplasms; n - number of patients; PV - polycythemia vera
* Statistically significant differences - χ2 test
Table 5 MPN-10 score differences between assessments (baseline vs. last follow-up visit)
ET - essential thrombocythemia; FU - follow up visit; MF - myelofibrosis; MPN - myeloproliferative neoplasms; n - number of patients; PV - polycythemia vera; SD - standard deviation
* Statistically significant differences - t-test
MPN
There were 43 patients with MF, 24 males, and 19 females. The male to female ratio was 1.3:1, the age ranged from 54 to 93 years old, median age 73 years, and median disease duration 7 months. The median Hb level was 9.8 g/dL (7.1-15.8 g/dL), the median HTC was 32.4% (22.0-46.7%), and the median PLT was 189.5 × 109/L (67.0-735.0 × 109/L).
There were 79 patients with PV, 38 males, and 41 females. The male to female ratio was 0.9:1, the age ranged from 32 to 92 years old, median age 73 years, and median disease duration 50.5 months (> 4 years). The median Hb level was 14.3 g/dL (9.5-17.8 g/dL), the median HTC was 44.7% (36.0-52.6%), and the median PLT was 272.0 × 109/L (8.3-720.0 × 109/L).
There were 201 patients with ET, 75 males, and 126 females. The male-to-female ratio was 0.6:1, the age ranged from 32 to 96 years old, median age 70 years, and median disease duration 43 months (> 3 years). The median Hb level was 13.2 g/dL (7.4-17.4 g/dL), the median HTC was 40.4% (0.3-53.9%), and the median PLT was 477.0 × 109/L (132.0-1,330.0 × 109/L).
No differences in laboratory parameters between baseline and last FU visit were observed, except for PV patients, whose mean Hb at baseline statistically differ from last FU visit, although without clinical significance (14.4±1.6 vs. 13.2±2.2 g/dL; p=0.006) (Table 2).
Therapy
At baseline, 253 patients had no support therapy (78.1%); 21 were on erythropoietin (6.5%), 6 were transfusion dependents (1.9%), and 47 were on other support therapy (14.5%). Most patients (n=283; 87.6%) were under pharmacologic treatment for MPN (>90% of PV and ET patients, 55.8% MF patients; p<0.05), 70.4% were treated with hydroxyurea (>70% of PV and ET patients, 30% MF patients, p<0.05), 30.6% were on recommended low-dose aspirin (ASA) (≈30% of ET and PV patients, 11.6% of MF patients, p=0.013), 9.0% needed phlebotomy (30% of PV patients and <5% of MF and ET patients, p<0.05), 3.7% received ruxolitinib (20.9% of MF patients, <1.5% of PV and ET patients, p<0.05), 1.5% interferon (≤2% of ET and PV patients, none of MF patients, p=0.614), and 5.6% other treatment (7% of MF and ET patients, 1.3% of ET patients, p=0.158) (Table 3).
MPN symptoms
Symptoms were evaluated by MPN Symptom Assessment Form (MPN-10) (Tables 4 and 5, Figures 1 and 2).
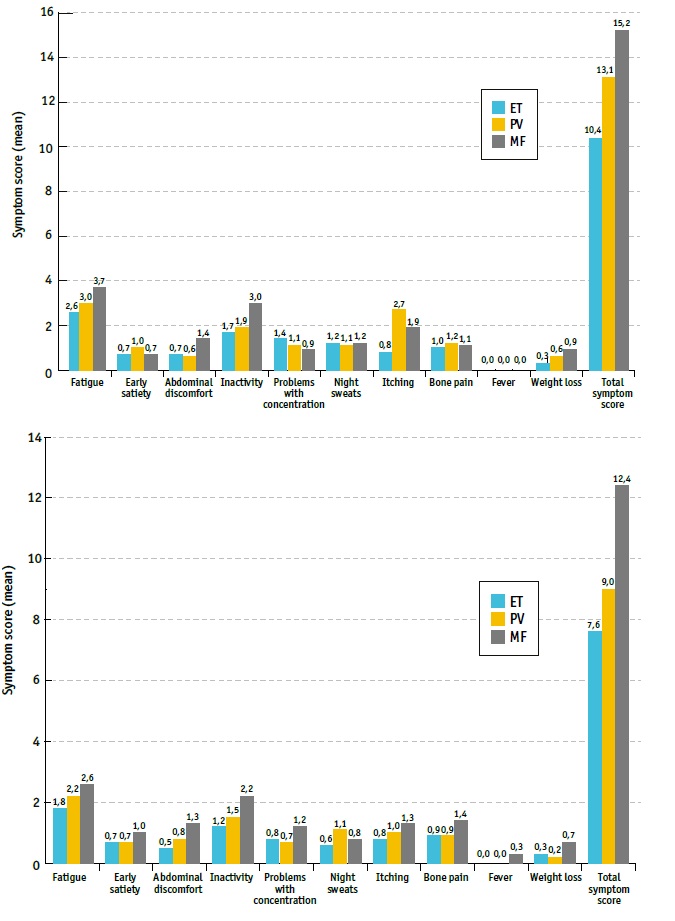
Fig 1 MPN-10 assessment tool results at baseline and last follow up visit, for ET, PV and MF patients. ET - essential thrombocythemia; MF - myelofibrosis; PV - polycythemia vera
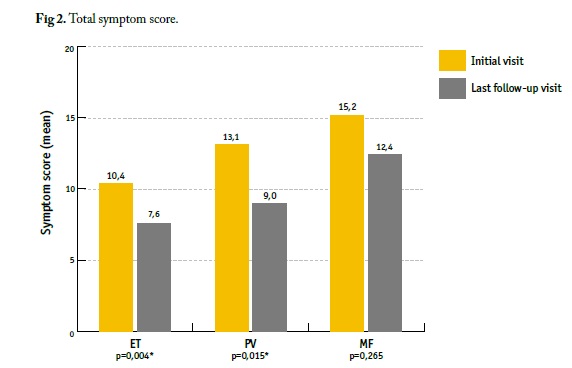
Fig 2 Total symptom score. ET - essential thrombocythemia; MF - myelofibrosis; PV - polycythemia vera. * Statistically significant differences
At baseline, most reported symptoms by MPN patients were fatigue (65.0%), with the highest mean severity of all reported symptoms among all MPN subtypes, followed by inactivity (47.4%), itching (35.3%), and concentration problems (34.1%) (Table 4, Figure 1). Fatigue (86.0%) and inactivity (67.4%) were the most reported symptoms in MF; fatigue (67.1%), itching (58.2%), and inactivity (45.6%) in PV; and fatigue (59.7%), inactivity (43.8%), and concentration problems (39.3%) in ET (Table 4). Although symptoms were generally present in all MPN subtypes, itching was notably more burdensome in patients with PV, while inactivity was the second most severe symptom reported by MF patients (Table 5, Figure 1).
Total symptom score improved from baseline until last FU, mean (±SD) MPN-10 score at baseline (initial visit) vs. last follow-up visit (FU) was 15.2±13.3 vs. 12.4±15.5 (p=0.265); 13.1±11.3 vs. 9.0±10.7 (p=0.015); 10.4±10.6 vs. 7.6±11.7 (p=0.004) in MF, PV, and ET patients, respectively (Table 5, Figures 1 and 2). Regarding each symptom, itching had a significant improvement towards baseline in PV patients (2.7±3.5 vs. 1.0±1.6, p<0.05), as well as fatigue (2.6±2.8 vs. 1.8±2.5, p=0.004), inactivity (1.7±2.6 vs. 1.2±2.2, p=0.022), concentration problems (1.4±2.4 vs. 0.8±1.8, p=0.001) and night sweats (1.2±2.3 vs. 0.6±1.5, p=0.001) in ET patients (Table 5, Figure 1).
Discussion
MPN form a group of rare hematological malignancies with different phenotypes and clinical presentations (A. Tefferi & Vardiman, 2008; Vannucchi & Guglielmelli, 2010; Verstovsek, 2010), associated with a reduced life expectancy compared with the general population (R. Mesa, Miller, et al., 2016; Petruk & Mathias, 2020; Roaldsnes et al., 2017). Nevertheless, MPN are chronic conditions for most patients, with median survivals of ≈33 years for ET, 24 years for PV, and 15 years for younger MF patients (<60 years old) (McMullin & Anderson, 2020; Petruk & Mathias, 2020; Shallis et al., 2020; Ayalew Tefferi et al., 2014).
Older age and male gender are known risk factors for MPN (Shallis et al., 2020). Additionally, males have higher PV and MF incidence rates, while females have higher incidence of ET (Byun et al., 2017; Deadmond & Smith-Gagen, 2015; Moulard et al., 2014; Roaldsnes et al., 2017; Shallis et al., 2020). Yet, and although a slight bias of MPN for males over females is often referred in the literature (Anderson et al., 2012; Deadmond & Smith-Gagen, 2015; Heppner et al., 2019; Moulard et al., 2014; Shallis et al., 2020; Titmarsh et al., 2014), the gender distribution in this population, as well as the distribution of patients per MNP subtype and mean age, were consistent with previous data regarding a Portuguese population (Ana P. Azevedo et al., 2017, 2018; Ana Paula Azevedo et al., 2017) and other studies and reports worldwide (Duncombe et al., 2020; Roaldsnes et al., 2017; R. Scherber et al., 2011; Seguro et al., 2020). In fact, ET and PV consistently make up the majority of MPN cases in formerly studied populations, with a median age at diagnosis between 50-60 years old, whereas MF median age at diagnosis is between 69 and 76 years old (Byun et al., 2017; Deadmond & Smith-Gagen, 2015; Duncombe et al., 2020; Heppner et al., 2019; Moulard et al., 2014; Roaldsnes et al., 2017; Titmarsh et al., 2014). Accordingly, in the present study, the median age of MF and PV was 73 years old, while the median age of ET was 70 years old, median age at diagnosis higher in MF patients than PV or ET. PV was associated with the highest mean Hb level (14.2 ± 1.6 g/dL), while MF showed the lowest (10.1 ± 1.8g/dL), which is coherent with the literature (Arber et al., 2016; Barbui et al., 2011; Byun et al., 2017). Likewise, patients with ET had higher PLC (530.3 ± 247.4 x 109/L), while a higher HTC (43.7 ± 4.3%) was found in PV patients (Arber et al., 2016; Barbui et al., 2011).
Despite therapeutic advances, nearly all treatment options for MPN are palliative, and thus, reduction of symptom burden and MPN associated impact on patients’ QoL should be considered a major treatment goal (Abruzzese et al., 2018; Petruk & Mathias, 2020). Current therapy in PV and ET aims at preventing thrombo-hemorrhagic complications, present strategies include cytoreductive agents, phlebotomy, and low-dose aspirin; and to alleviate anemia, symptomatic splenomegaly, or constitutional symptoms in MF (Barbui et al., 2011; Seguro et al., 2020). Accordingly, >70% PV and ET patients were on hydroxyurea vs. 30% MF patients; 30% PV and ET patients were on ASA vs. 12% MF patients; 30% PV patients underwent phlebotomy vs. <5% ET and MF; and 20% MF patients were on ruxolitinib vs. <1.5% PV and ET patients.
Patients with MPN experience a broad array of symptoms, which include microvascular-related symptoms, systemic manifestations (fatigue, night sweats, insomnia, body weight loss, and fever), pruritus, and splenomegaly, with associated abdominal complaints and early satiety. The overall burden of disease has a substantial negative impact on patients’ lives. Although the impact is generally most significant in MF patients, in higher-risk patients or those with greater symptom burden, negative effects are seen across the disease spectrum (Anderson et al., 2015; Brochmann et al., 2019; Harrison et al., 2017; McFarland et al., 2018; R. Mesa et al., 2018; R. Mesa, Miller, et al., 2016; Petruk & Mathias, 2020; Yu et al., 2018, 2019). To provide patients with the best care, due to the complexity of care, follow-up necessity, and symptom control, a multidisciplinary team of specialty-trained oncology nurses, oncologists, pharmacists, and additional support staff, should be involved.
The MPN-10 is a valid and reliable instrument that concisely assesses the prevalence and severity of MF, PV, and ET symptoms. Results from serial survey administration indicate that the MPN-10 captures both the primary disease state and continued disease presence, even amid standard therapy treatment (R. Scherber et al., 2011). In addition to being well validated, the MPN-SAF is a tailored measurement tool whose evaluation is based on patient reported outcomes (PRO) and comprehensive assessment of MPN symptom burden. PRO measurements represent a subjective assessment of self-reported symptoms and treatment effects that have been found to be useful in guiding critical clinical decisions, particularly when objective evaluation of physical manifestations is difficult (R. Scherber et al., 2011). Other PRO instruments do not measure the breadth of symptoms specific to MPN disease, such as abdominal pain, bone pain, headache, pruritus, weight loss, fever, and cough (R. Scherber et al., 2011). In common with other patient surveys, the most frequently reported and severe symptom was fatigue (Anderson et al., 2015; R. A. Mesa et al., 2007; Petruk & Mathias, 2020; R. Scherber et al., 2011). Other commonly reported symptoms varied depending on disease subtype: inactivity, itching, concentration problems, bone pain, and night sweats. As expected, MNF-10 total symptom score was higher for MF than for PV and ET (R. Scherber et al., 2011). Total symptom’s score significantly improved from baseline until last FU visit for each MPN subtype, itching (notably more burdensome in patients with PV), had a significant improvement towards baseline in PV patients, as well as fatigue, inactivity, concentration problems and night sweats in ET patients.
Conclusion
The symptomatic burden is present in most patients with MPN compromising patients’ QoL. PROs and QoL measures may improve disease monitoring and management, being essential for improving MPN patients’ outcomes. Despite their importance, real-world data are generally limited. The systematic application of the MPN-10 scale, a reliable and inexpensive instrument recommended by the NCCN guidelines, led to a more in-depth knowledge of these patients and their symptoms, which, together with analytical changes, motivated a therapeutic readjustment that led to gains in QoL. Although these results reflect Portuguese reality regarding patients with MPN, future analysis of this cohort should be conducted to refine these results and better understand and manage the disease.
Acknowledgments
The authors would like to thank the patients who have agreed to participate in this study. This study was developed by the Associação de Enfermagem Oncológica Portuguesa (AEOP) with the financial support of Novartis.
Ethics approval
This study was approved by the institutional review boards or ethics committees of the study sites.
Consent to participate and consent for publication
Informed consent to participate in this study and to have the data published was obtained from all individual participants involved in the study.