Introduction
Radiocontrast media (RCM) are within the most used pharmacological agents nowadays since they increase sensitivity and specificity in diagnosis. RCM are mainly divided into two categories: iodinated contrast media (ICM), which are classified according to its benzene tri-iodate ring, and gadolinium-based contrast agents (GBCA). The first ever radiology exam described using RCM was an angiography in 1920.1 In 1988, after approval of the first gadolinium-based contrast agent by the US Food and Drug Administration (FDA), gadopentetate dimeglumine, the use of this contrast agent increased and multiplied worldwide, as it enhanced the quality and specificity of radiology exams, a major contribution to the modern diagnosis. Annually, over 75 million of procedures require GBCA.2
In accordance with the incremental use of RCM, specially GBCA, required in magnetic resonance imaging (MRI) examinations, the description of hypersensitivity reactions to GBCA has been increasing. The frequent misrecognition of symptoms, combined with the absence of mandatory registration, leads to a challenging and often delayed diagnosis, and to a still unknown real prevalence of these reactions.
Adverse events following the administration of dose adjusted GBCA can be divided into three different types: hypersensitivity reactions, toxic reactions and events unrelated to the exposure of contrast material itself. According to the literature, the incidence of acute adverse reactions after intravenous administration of GBCA ranges from 0.07-2.4%.3 However, only 0.004-0.7% corresponded to hypersensitivity reactions.4
Hypersensitivity reactions can be classified as immunological, IgE mediated or non-IgE mediated, and non-immunological. The most frequent reactions described for GBCA are immediate reactions, the majority of which classified as mild, where skin manifestations, such as urticaria, pruritus and angioedema, are presented in 75-100% of cases.5 The rate of recurrence for these reactions is about 30%.6 Although most hypersensitivity reactions described are non-allergic, in case of a severe reaction it is more likely to be IgE mediated.7 Even though, anaphylaxis and mortality related to hypersensitivity reactions for GBCA are rare, its incidence reaches 0.01% and 0.0019%, respectively.8,9Accordingly, an early recognition, registration and management of these reactions are cornerstone to provide adequate medical care.
In immediate hypersensitivity reactions, occurring within the first six hours after injection, whether IgE mediated or non-mediated, the degranulation of mast cells and basophils leads to the release of histamine and other vasoactive mediators responsible for symptoms such as nasal congestion, bronchospasm, dyspnoea, hypotension, tachycardia, nausea, vomiting and diarrhoea. In non-IgE mediated reactions the occurrence of such symptoms relies on the direct effect of the contrast on the cell membrane, leading to mediator's release, activation of the complement cascade and/or bradykinin release. Non-IgE immediate reactions tend to occur within the same day of exposure in 46% of cases and in the day after in 20% of cases.10
Considering the risk factors for developing adverse reactions to GBCA, the occurrence of a previous documented reaction, whether to an ICM or distinct GBCA, is the major risk factor. Female gender, antecedents of atopy, such as asthma, allergic rhinitis, chronic urticaria, food allergies and drug hypersensitivity, underlying chronic diseases such as cardiac, renal and hepatic, as well as diabetes mellitus and use of beta-blockers also play an important role in terms of increased incidence of hypersensitivity reactions, representing relevant determinants factors.5,6Even though clonal mast cell disorders have been advocated as a risk factor11, recent studies have showed that they did not affect the incidence of hypersensitivity reactions, as previously thought.6,12Independently, none of these conditions are an absolute contraindication to the use of GBCA.
As gadolinium administered directly is toxic, all GCBA need in their composition a chelating compound, of linear or cyclic morphology, to provide its solubility and safety. The classification of GBCA is made upon the molecular structure of the used chelating compound (linear or macrocyclic) and according to its charge when in aqueous solution (ionic or non-ionic). Linear GBCA include Gadopentetate dimeglumine - Magnevist®, Gadobenic acid - MultiHance®, Gadoxetate disodium - Primovist®, ionic agents, and Gadodiamide - Omniscan®, a non-ionic agent. Concerning the macrocyclic GBCA, they include Gadoteridol - ProHance® and Gadobutrol - Gadovist®, non-ionic, and Gadoteric Acid - Dotarem®, ionic.7,13These agents are mainly extracellular and present renal excretion and, in little extent, hepatic excretion.7,14,15
We aimed to characterize clinically and demographically a population with previous history of reaction to GBCA, evaluate the sensitization profile to GBCA and identify possible sensitization profiles associated with severe reactions.
Material and Methods
Study population
We conducted a retrospective analysis of the patients followed in the outpatient Allergy Clinic of a tertiary hospital, between January 2014 and June 2022, for suspected hypersensitivity reactions to GBCA.
We enrolled 36 patients with a suggestive clinical history of hypersensitivity to GBCA. Data regarding gender, age, time from reaction, atopy, comorbidities, culprit contrast media, clinical manifestations and treatment was collected from the clinical records. Previous exposure to contrast agents and the administered premedication were analysed. The severity of the adverse reactions was classified based on the Ring and Messmer Classification (Grade I-IV) and were considered immediate whenever they occurred within the first six hours after the GBCA administration. Delayed or nonimmediate reactions were described concerning the reported symptoms.
All patients were included in the study, regardless of missing data.
The study followed the recommendations of the Ethics Committee and of the World Medical Association (Declaration of Helsinki revised in 2013) and informed consent was obtained from patients.
Allergy study
Skin tests were conducted in conformity with the European Academy of Allergy and Clinical Immunology/European Network for Drug Allergy recommendations.16 In case skin prick tests (SPT) with undiluted commercial solutions were negative, intradermal tests (IDT) to GBCA diluted at 1:1000, 1:100 and 1:10 in 0.9% sterile saline were performed, with immediate (20 minutes) and delayed (24 and 48 hours) readings. In the case of severe reactions, IDT began with higher dilutions. Investigation with patch tests was also pursued in selected cases and followed the European Society of Contact Dermatitis recommendations.17
Contrast media
The following gadolinium-based contrast media were used during the study period: macrocyclic agents, ionic gadoteric acid (Dotarem®, 279.32 mg/mL) and non-ionic gadobutrol (Gadovist®, 1 mmol/mL); linear agents, non-ionic agent gadodiamide (Omniscan®, 287mg/mL) and gadoxetate disodium (Primovist®, 0.25mmol/mL).
Statistical analysis
Descriptive analysis of demographic and clinical data was performed. Categorical variables are presented as absolute (n) and relative frequencies (%), and continuous variables as mean ± standard deviation (SD) (minimum and maximum) and median and 1st and 3rd quartiles (Q1-Q3), except when mentioned otherwise. All descriptive statistical analyses were performed using GraphPad Prism 5® (version 5.03).
Results
Demographic and clinical characterization
During the 8-year period, 37 reactions to GCBA were suspected in 36 patients referred to the outpatient Allergy Clinic. The study population included mostly female patients (n= 32, 88.9%) within adult age (mean 54.1 ± 16.0, minimum 27 - maximum 89 years). Table 1 summarizes the baseline clinical characteristics of the study population. Of note, almost one third of the patients presented a previous history of non-RCM drug allergy (n=13, 36.1%), 12 patients had a previous exposure to RCM (33.3%), four of whom with a reported preceding adverse reaction (11.1%). The first Drug Allergy appointment occurred 4.7 ± 7.0 years after the adverse event.
Detailed characterization of hypersensitivity reactions to GBCA is shown in Table 2.
Table 1: Clinical characterization of patients with hypersensitivity to gadolinium-based contrast agents.
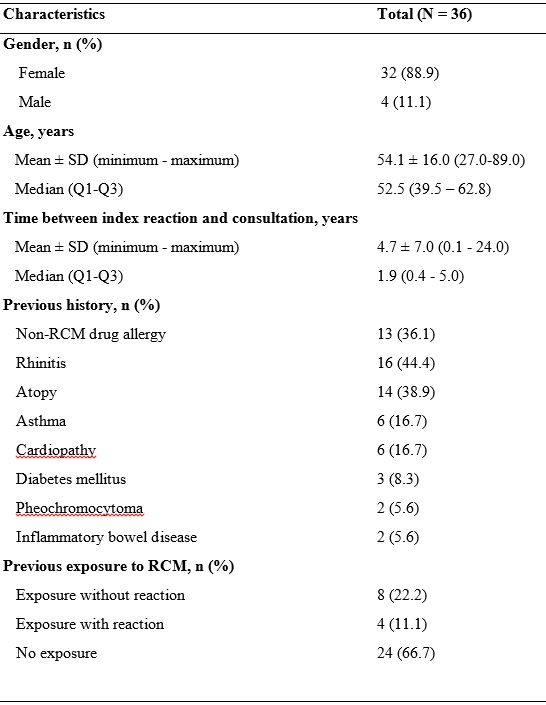
N, total number of patients; Q1, 1st quartile; Q3, 3rd quartile; RCM, Radiocontrast Media; SD, standard deviation.
Table 2: Detailed characterization of hypersensitivity to gadolinium-based contrast reactions.
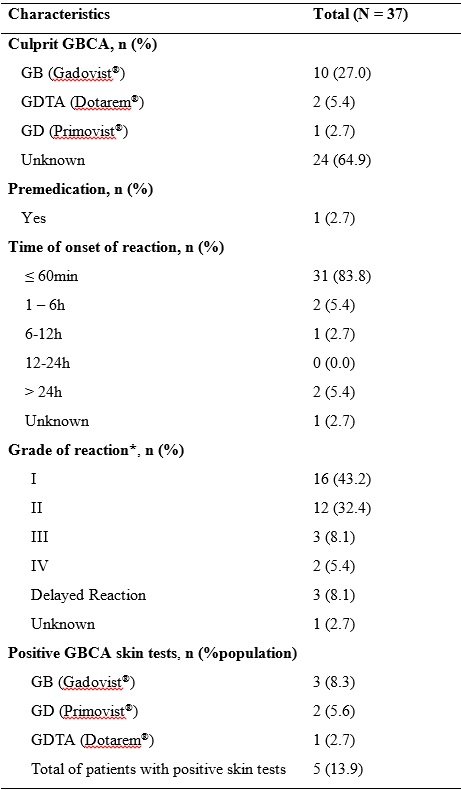
GB, Gadobutrol; GBCA, Gadolinium-based contrast agent; GD, Gadoxetate disodium; GDTA: Gadoteric acid; N, total number of patients; Q1, 1st quartile; Q3, 3rd quartile; SD, standard deviation.
*According to Ring and Messmer Grading Scale.
Culprit GBCA agent
The most common culprit GBCA agent identified was gadobutrol in ten cases, followed by gadoteric acid in two and gadoxetate disodium in one patient. In 24 events (64.9%) the culprit GBCA was not determined. All the GCBA were administered intravenously.
Premedication
Only one patient received premedication (corticosteroids) previous to the adverse event, due to a history of cardiopathy, atopy (allergic rhinitis), as well as non-RCM drug allergy (beta-lactam antibiotics). Nonetheless, the patient reported a maculopapular rash 120 minutes after the GBCA administration.
Clinical manifestations and management
Among the 31 patients who presented immediate hypersensitivity reactions, grade I reaction was the most common, reported in 16 reactions (43.2%), followed by grade II in 12 reactions (32.4%), grade III in three (8.1%) and grade IV in two reactions (5.4%).
The individuals with the most severe reactions were women, age 86 and 42, and both had an oncologic history (lung cancer and leukaemia). Additionally, one presented cardiac disease. No other risk factors were identified and there was no known previous exposure to contrast media.
Delayed reactions were described in 3 cases and occurred on the same day (n=1), on the second (n=1) and on the fourth (n=1) day. Maculopapular rash was the most common manifestation (n=2), followed by delayed urticaria with angioedema (n=1).
The majority of the reactions were treated with corticosteroids and antihistamines (n=14, 37.8%), four reactions with antihistamines (10.8%), three were treated with corticosteroids (8.1%) and four required adrenaline (10.8%). The two previously described patients, reporting the most severe reactions, required advanced life support. No fatalities occurred during the study period.
Allergological work-up
The allergological work-up documented positive GBCA tests in five patients (13.9%) during the 20-minute reading. SPT were positive in five patients: gadobutrol (n=3) and gadoxetate disodium (n=2). One patient with positive SPT gadobutrol result also presented positive IDT to gadoteric acid. All the positive tests corresponded to patients that developed immediate hypersensitivity reactions: two patients experienced an immediate reaction grade I, two patients a reaction grade II and one patient a grade IV event. Adrenaline treatment was required in two patients. Only one patient had a previous exposure with RCM, which was tolerated.
Patch tests were performed in seven patients, without positive results.
Drug provocation tests and re-exposure analysis were not performed.
Discussion
Gadolinium is a heavy metal with paramagnetic properties, used in intravascular or intraluminal studies. By itself, it is toxic, so it needs to bind a chelator to increase the safety and solubility of its core ion. Thus, GBCA are then divided into linear or macrocyclic and ionic or non-ionic.7,13
Hypersensitivity reactions to RCM and, specifically to GBCA, are more frequent in adulthood, and this was also verified in our study, where the sample consisted only of adult individuals.7,13,15
The existence of a previous reaction is the main risk factor described for developing a hypersensitivity reaction to GBCA, increasing the risk to almost 60%, as is the case with ICM. One third of our sample had already been exposed to RCM, 22.2% with reaction.6,7,10,13,14,15
Other predominant factors also described include: history of drug allergy (other than RCM allergy), female gender, atopy and presence of other comorbidities, such as cardiovascular diseases or renal function impairment. In our cohort, the vast majority of patients were female (88.9%), in accordance with the literature. Also, drug allergy (36.1%), atopy (14%) and cardiovascular diseases/diabetes mellitus (26.5%) have been described. The presence of these factors may have precipitated the reaction. Renal function before the exams and after the reaction was not evaluated.6,7,13,14,15
It should be noted the fact that a mean time of 4 years (in one patient it took 24 years) has passed since the reaction until the first specialized consultation, which demonstrates a significant delay in these patients’ referral to specialized centres in order to perform the investigation. The latest international guidelines advocate that skin tests should be performed between 2 to 6 months after the reaction, so it is very important to spread the need for investigation of these patients within the advised timing, fomenting their referral as soon as possible.6,7,10,14
Regarding the GBCA identified by the patients/radiology centres as the culprit one, gadobutrol was the most GBCA frequently identified in about one third of the patients, as predicted in other studies.5-9,13,14,15This can be explained either by the fact that a more appropriate and timely diagnosis of hypersensitivity reactions to RCM is being made, but also by the fact that gadobutrol is the GBCA most used worldwide, given its non-ionic macrocyclic characteristics. Nevertheless, it is important to emphasize that in more than two thirds of the sample it was not possible to identify the GBCA responsible for the reaction, thus making the diagnostic process difficult. It is important to sensitize the medical community to the need to identify the RCM used in imaging exams, in order to perform a correct diagnosis and find safe alternatives.
According to the literature, and unlike ICM, the most frequent occurrence of immediate hypersensitivity reactions is described for GBCA, which was verified in our population, where approximately 90% of patients developed symptoms in the first 6 hours after the administration of the GBCA.5-9,13-15
The same can be observed regarding the severity of the reaction. According to the literature, the most frequent GBCA hypersensitivity reactions described are mild, mainly with skin involvement only (75-100%): the majority of the patients in our study (77.8%) had mild reactions (grade I or II on the Ring and Messmer scale), being the skin the most frequent involved organ.5,7,8,14Furthermore, also the delayed reactions were mild, with only maculopapular rash and delayed urticaria.
Premedication with corticosteroid was administered to only one patient, which did not change the final outcome, as he reported a maculopapular rash two hours after GBCA administration. This fact is in line with what has been described about the low effectiveness of premedication with corticosteroids in the case of immediate reactions to GBCA, as opposed to reactions to ICM whose effectiveness has been demonstrated.4,7,14
Regarding the skin tests, it is recommended that they should be carried out only in patients with previous reactions, not only to avoid sensitization during the procedure, but also because they do not predict the risk of future reactions in patients without previous reactions, even in the presence of risk factors. In our study, all patients performed skin tests as all were referred for suspected reaction to GBCA.6,7,13,14
Only five (14%) patients had positive skin tests, all reporting an immediate reaction, which is in accordance with previous studies that describe a sensitivity of 4.2% to 73% in skin tests for immediate reactions. This can be due to the fact that almost all patients were referred to our specialized centre within a median time of 2 or more years after the reaction, delaying the investigation with a consequent decrease in skin reactivity.7,13,14Positive skin tests were mainly with gadobutrol, as it was the most frequent GBCA identified as the culprit, but also with gadoxetate disodium; in only one patient gadobutrol was suspected to be the culprit GBCA, in the remaining, the culprit GBCA was not known. The fact that one patient showed positivity with gadobutrol and gadoteric acid, both macrocyclic GBCA could express some cross-reactivity, that is described as more often with macrocyclic GBCA, manly when gadobutrol is involved. Linear GBCA show less degree of cross-reactivity, although their use is becoming more obsolete due to the risk of tissue deposition (brain and kidney).7,14
Drug provocation tests were not performed, as they are only recommended when no alternative is available, due to their potential and harmful side effects, such as kidney disease. It was recommended that all patients should avoid the culprit GBCA, when known, or those positive on skin tests.3,7,13
The main limitation of our study is that it was a retrospective observational study based on patients’ reports, sometimes from reactions that occurred many years ago, which can have a significant memory bias.
Conclusion
Currently there are scarce studies on hypersensitivity to GBCA, so the allergy work-up keeps representing a challenge, being crucial to decide the clinical approach for each patient and try to stablish the culprit drug or alternative ones, so the patients can perform their MRI safely. For this reason, we believe that our data give us added value, increasing our knowledge about GBCA hypersensitivity reaction in a Portuguese population.
Even though anaphylaxis and mortality related to hypersensitivity reactions for GBCA are rare, early recognition and management of these reactions are cornerstones to provide adequate medical care.
We found that most reactions were immediate, mild and mainly cutaneous, with less severity. The most frequent GBCA involved was gadobutrol, as it is the most widely used GBCA, as described in previous studies.
We identified a culprit GBCA in five patients and could allow the use of safe alternatives in all patients, thus highlighting the importance of a correct diagnosis and selection of an alternative drug.
Our study has an important added value in clinical practice, providing real-world evidence for the scientific community about the clinical characteristics of patients with suspicion of GBCA hypersensitivity, being the largest series described from a single center so far in Portugal.















