Case Report
A 79-year-old man was referred to our institution with a history of painless, long-lasting and insidious swelling in the right parotid region, worsening in the previous three months. He had no relevant medical history. At physical examination, he had a facial asymmetry, with a right parotid painless, indurated mass measuring about 6 cm, extending anteriorly to the masseteric region and posteriorly to the retro-auricular area. The mass was also associated with notable irregular cutaneous violaceous plaques (Fig. 1).
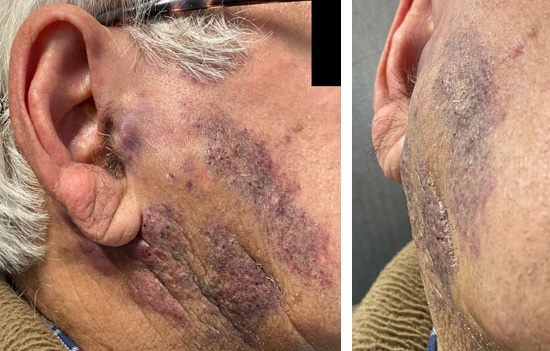
Figure 1: Right parotid region swelling associated with irregular violaceous skin plaques, extending anteriorly to the masseteric region, and posteriorly to the retroauricular region.
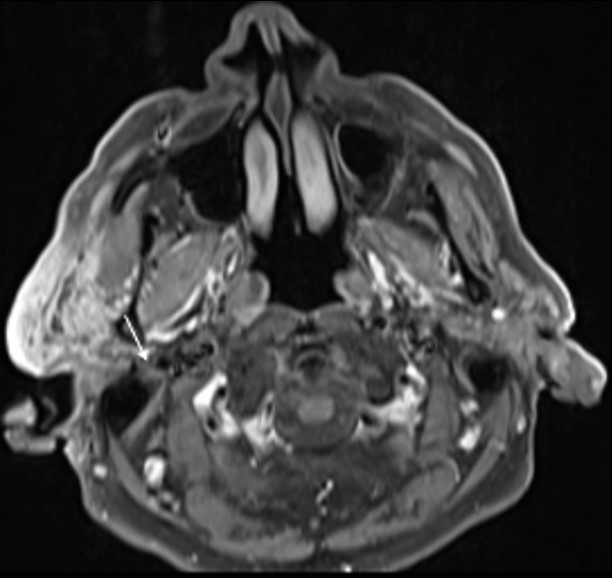
The patient denied a history of recent trauma. There was no evidence of facial paralysis or pain during palpation. A painless fibroelastic lymph node of about 1 cm was apparent at the right cervical level II. Facial/Cervical magnetic resonance imaging (MRI) and cervicothoracic computed tomography (CT) were performed, showing a diffuse infiltrative process of the right parotid gland, mimicking an inflammatory process (Fig. 2 and 3). The lesion showed a low signal on T1 and a heterogeneous signal on T2-weighted images, with heterogeneous post-contrast enhancement.
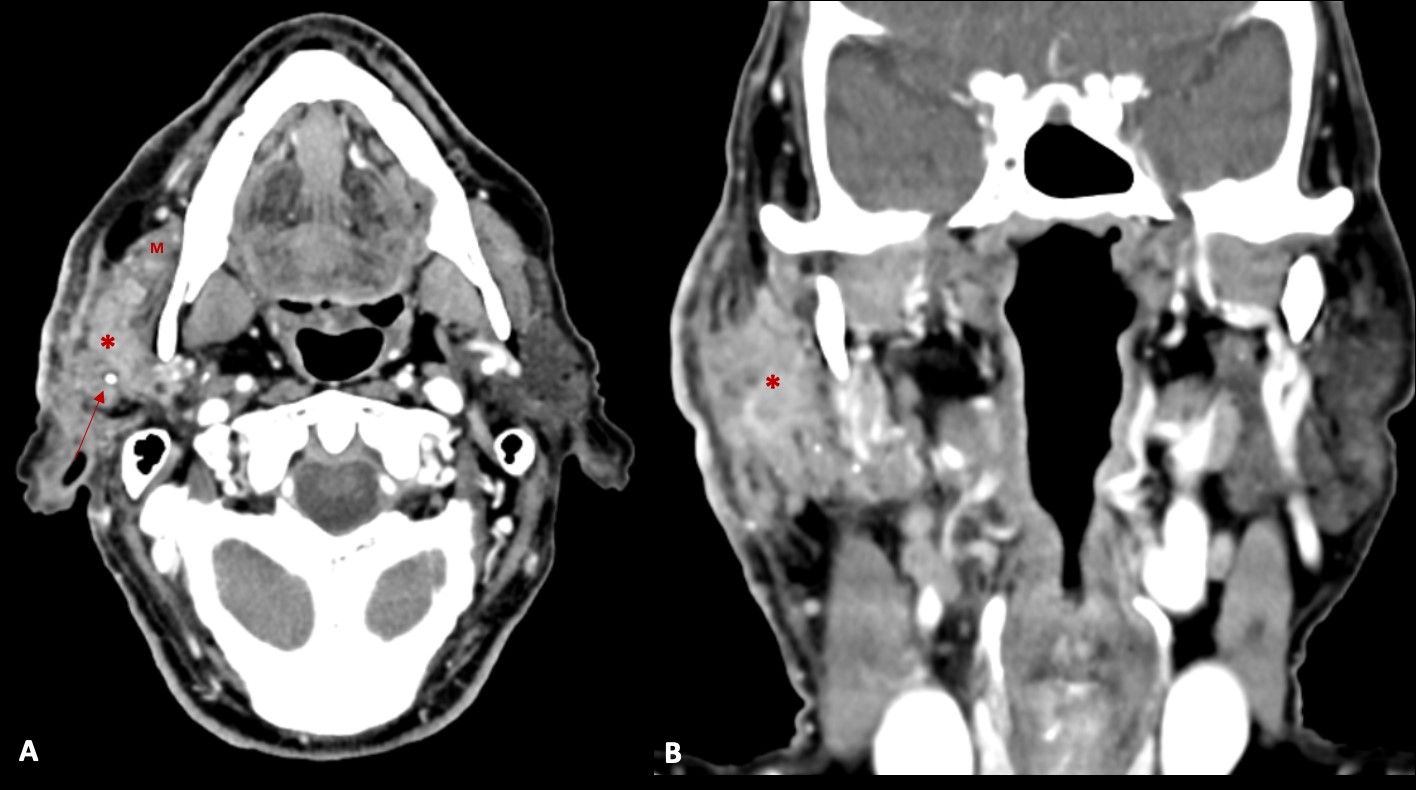
Figure 2: Contrast enhanced axial (A) and coronal (B) CT images, demonstrating an infiltrative mass (*) in the right parotid gland (in both superficial and deep lobes), that infiltrates the surrounding fat planes and skin, and invades the right masseter muscle (M). Some calcifications (arrow) are also present.
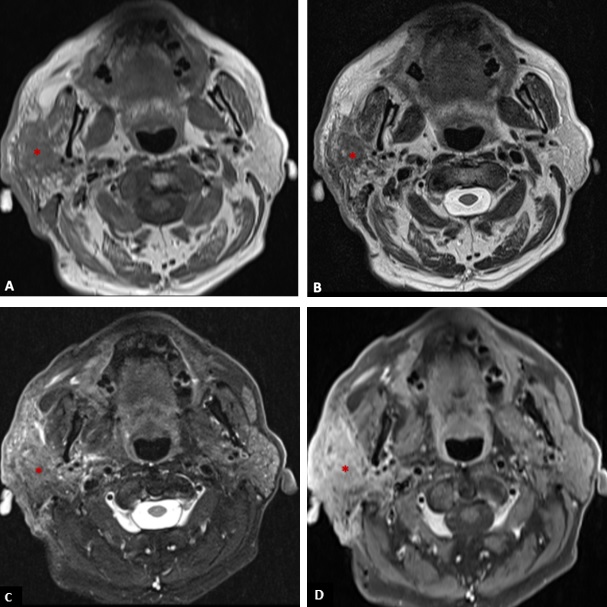
Figure 3: T1-weighted axial image (A), T2-weighted axial image (B), fat suppressed T2-weighted axial (C), and post-contrast fat suppressed T1-weighted axial image (D) showing an infiltrative mass (*) in the right parotid gland with low signal on T1, heterogeneous signal on T2 (with areas of high and low signal), and heterogeneous enhancement invading the adjacent fat, skin and the right masseter muscle.
The lesion also revealed restricted diffusion, with an ADC value of 0,858 x 10-3 mm2/s (Fig. 4). Additionally, a more focal nodular and irregular area was evident in the superficial parotid lobe. There was also a noticeable infiltration of the adjacent fat planes and skin, invasion of the right masseter muscle, and extension of the lesion to the submandibular space without the involvement of the right submandibular gland (both submandibular glands were atrophic). The right facial nerve showed a moderate high signal on T2-weighted images and post-contrast hyperenhancement that was perceptible in its path up to the stylomastoid foramen, which was a cause of concern regarding perineural spread (Fig. 5). Retromandibular vein thrombosis was visible. An increased number of lymph nodes was present in all right cervical levels, the largest node measuring 11 mm (in greater diameter), some of them showing round morphology and central areas of necrosis. There were no signs of mandibular invasion or other suspicious bone lesions. CT also excluded signs of lung metastases. Ultrasound-guided fine needle aspiration of the right parotid lesion was performed, demonstrating involvement of the parotid parenchyma by a malignant tumor with a nest pattern constituted by epithelioid cells with eosinophilic cytoplasm and atypical nuclei, with evident nucleolus and frequent mitoses. Comedonecrosis-type tumor necrosis areas, as well as lymphatic and vascular invasion were observed. The findings were compatible with a salivary duct carcinoma (SDC), with expression of androgen receptors. The management options were discussed in a multidisciplinary group meeting and with the patient. Due to the extension of the neoplastic lesion and the potential morbidity associated with the surgical procedure that would preclude a complete resection, radiotherapy and antiandrogen therapy were chosen.
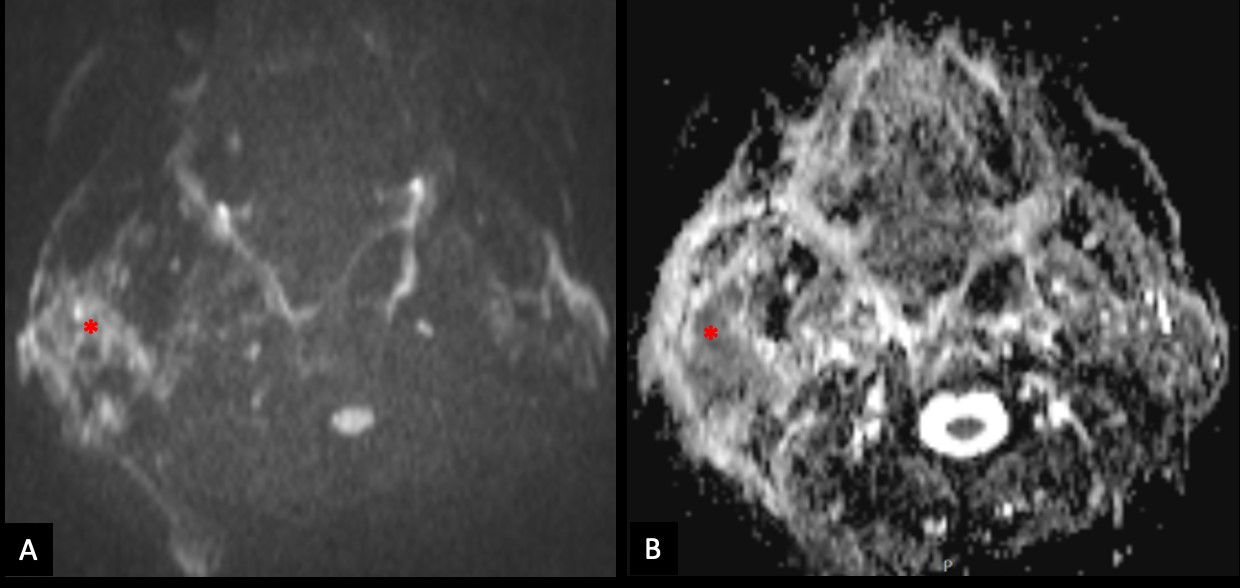
Figure 4: The right parotid lesion shows moderate restricted diffusion (*), with high signal on diffusion-weighted image with b value of 800s/mm2 (A) and low signal on apparent diffusion coefficient map (B).
Discussion
SDC is a rare and highly aggressive adenocarcinoma that accounts for 1-3% of all malignant salivary gland tumors.1,2,3It was first described by Kleinsasser et al. in 19684 and later refined by others, and about 200 cases have been reported in the English literature. The name results from its pathomorphological similarities with mammary duct carcinoma,5 which arises from the salivary gland's ductal epithelium.3 SDC accounts for 0.9-6% of all parotid tumors, which is the most commonly involved salivary gland,6 while submandibular and minor salivary glands are rarely concerned. It often involves the extrapetrous portion of the facial nerve and tends to metastasize through the temporal bone via perineural spread.7 Gingival metastases have also been reported.8 It may develop on the basis of a pre-existing pleomorphic adenoma, but it can also reoccur. In our case, the presence of a nodular component in the superficial lobe of the parotid might raise the possibility of a pre-existing pleomorphic adenoma. Patients are usually older men in their 7th decade of life,9,10with male-to-female ratios of 5.5:1−7.7:1.3 The lesion presents as a rapidly growing mass, which develops aggressively with serious risk of early distant metastases, local recurrence, and high mortality. Facial paralysis is observed in 40-60% of cases, and lymphadenopathies in 35% of cases.10 Reports on the radiologic features of salivary ductal carcinoma have been limited in the literature. Imaging findings, especially CT and MRI features, are nonspecific but helpful in the diagnosis of malignancy, in the management of these patients, and also for staging. Imaging can indicate the malignant nature of the tumor by showing ill borders or infiltration of the adjacent tissues. On CT scans, the lesions often appear as an ill-defined infiltrative mass with calcification and necrosis. At MRI, the signal intensity of the tumor may vary on both T1- and T2-weighted images. On T2-weighted images, these tumors often show heterogeneous signal intensity. MR imaging has been widely used to differentiate between benign and malignant tumors of the salivary glands, as the lower the signal intensity on T2-weighted images, the higher the possibility of high-grade malignancy. The low signal intensity on T2- weighted images reflects the pathologic features commonly seen in high-grade malignancies of the salivary glands, such as the lack of serous and mucinous products, high mitotic ratio and cellularity, and high nuclear-to-cytoplasm ratios. Some studies emphasize the low signal intensity of SDC on T2-weighted images, which seems to be in relation to areas of desmoplastic reaction associated with an infiltrating tumor. In contrast, areas of hyperintensity on T2-weighted images seem to correlate histologically with a mixture of various proportions of fibrosis, necrosis, comedonecrosis, tumor cells, and lymphoplasmacytic infiltration.3 These tumors also demonstrate lower signal intensity on T2-weighted images than on STIR images.9 In patients with high-grade salivary gland cancers such as SDC, 18F-FDG PET/CT is considered to have high accuracy in predicting the pathologic extent of primary tumors and extension to neck nodes, and it might be useful given the aggressive nature of these tumors, which frequently present with lymphatic spread at the time of diagnosis.11 The final diagnosis is based on histologic examination. The diagnosis may be obtained in several ways, namely through fine needle aspiration cytology (useful but not always reliable), core biopsy, or surgical specimen. Gross findings consist of a tumor of variable size, usually firm with a variable cystic component. Infiltration of the adjacent parenchyma is usually notorious. Microscopically, the most typical feature is the similarity with ductal carcinoma of the breast. The tumor comprises intraductal and invasive components, and comedonecrosis (central zone necrosis within a duct) is a frequent feature.9 Immunohistochemical findings reveal a constant overexpression of keratin, CEA, HER/2 neu, and c-erd-B2. The presence of androgen receptors and prostate-specific antigen expression have been frequently reported.8,12Differential diagnoses include mucoepidermoid carcinoma, adenocarcinoma not otherwise specified, metastatic adenocarcinoma, and oncocytic carcinoma. An important morphologic feature is the presence of an intraductal component, which is specific for the diagnosis. The therapeutic approach seems to be non-consensual because of the limited data. Many authors recommend a total parotidectomy in parotid gland tumors, even in T1-staged tumors, because local disease recurrence is often life-threatening.10,13In the presence of facial paralysis, a radical parotidectomy is mandatory. In the presence of involvement of the submandibular or minor salivary glands, lesion resection with wide margins is indicated to control the local disease.10 Regardless of its primary location, ipsilateral functional neck dissection is generally indicated because regional lymphatic spread has to be expected in most patients at the time of diagnosis. In the case of minor gland involvement, a bilateral neck dissection should be performed because lymphatic drainage may occur to the contralateral side. The effects of postoperative adjuvant radiotherapy are uncertain, but the aggressiveness of this tumor may justify the indication of adjuvant measures. Postoperative radiation therapy is suggested in case of extraparotid extension, pathological resection margins, cervical lymph node metastasis, lymphatic embolus, and perineural dissemination. Chemotherapy is usually reserved for metastatic forms of disease presentation.14 Kuroda et al. reported the utility of both anti-androgen therapy and chemotherapy in a patient with advanced SDC.15 In our case, the management consisted of radiotherapy due to the extension of the tumor and the morbidity associated with an eventual surgical intervention, as well as antiandrogen therapy due to the expression of androgen receptors. SDC is an aggressive tumor with a poor prognosis due to its metastatic potential. Local disease recurrences and distant metastases dominate the clinical course. After initial treatment, the overall life expectancy is 56 months. Prognostic criteria are non-consensual and consist of tumor size (superior to 3 cm), infiltrative tumor margins, local recurrence, lymphatic and distant metastases, necrosis, and percentage of infiltration. Involvement of the parotid gland seems to be related to a better prognosis than involvement of the other salivary glands.10 While that was the case in our patient, several findings were associated with a worse prognosis: tumor size, infiltrative tumor margins and the presence of lymphatic and vascular invasion.