Introduction
Neck masses in the paediatric age are relatively common, comprising various aetiologies, including congenital, inflammatory/infectious, and neoplastic lesions. The clinical history and physical examination are crucial in narrowing the differential diagnosis, with the imaging evaluation being fundamental to determine the definitive diagnosis and the extent of the lesion, helping to plan the most appropriate clinical or surgical approach.
Congenital masses are typically present at birth or discovered during the neonatal period. However, some may only present later either because of superimposed infection or continuous growth over time. Inflammatory lesions usually present as a rapidly growing mass with associated fever, tenderness, and overlying erythema. History of a recent head and neck infection, such as an upper respiratory tract infection, suggests possible reactive lymphadenopathy or a secondary infection of a congenital cyst. Exposure history is also important to assess for animal exposure, contact with other sick children or adults, and recent foreign travels. Neoplastic malignant lesions are rare in children, representing 11-15% of all neck masses1, however, these must be considered if a new enlarging, persistent (>6 weeks) mass appears.2 These are frequently asymptomatic, not painful, but can be associated to constitutional type B symptoms (fever, malaise, weight loss, night sweats), which should raise suspicion. Neoplastic benign lesions frequently present as slowly enlarging masses over months to years, some being developmental masses.
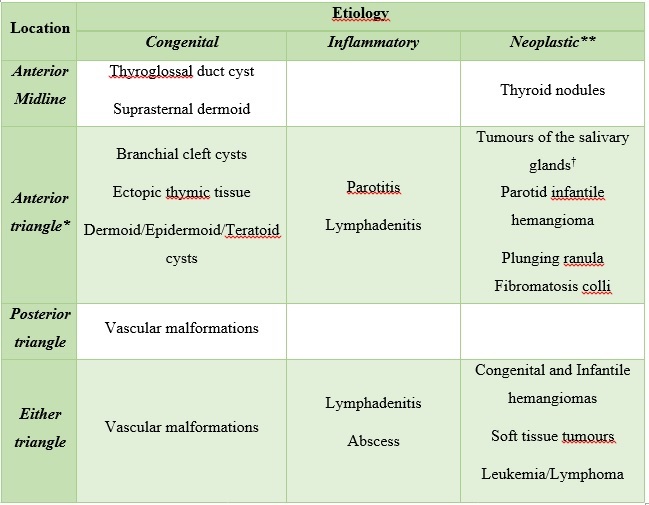
On clinical examination is important to note the location and the characteristics at palpation of the lesion. Some lesions occur specifically in the anterior or posterior neck triangle, while others can present in either compartment. Regular and mobile lesions suggest a benign origin, contrary an irregular, adherent and non-tender lesion which is suspicious for malignancy. To facilitate the reader search, we organized neck masses by location and aetiology(Fig. 1) (Tabela 1).
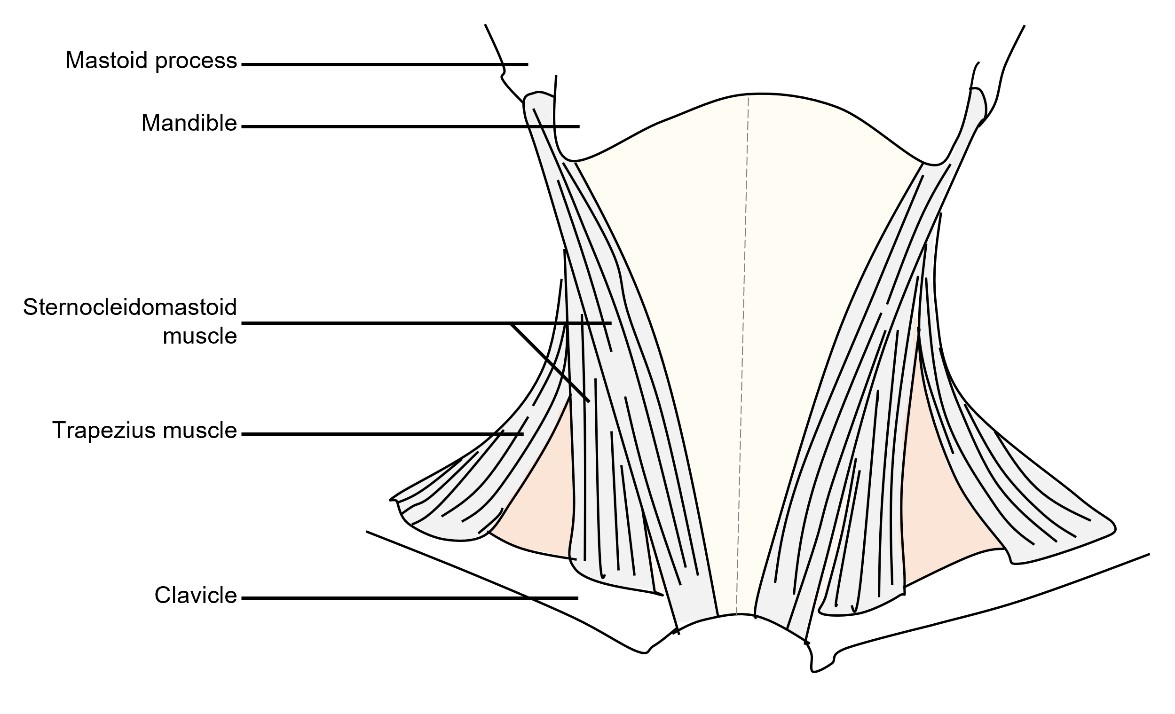
Figure 1: Cervical neck triangles. The anterior cervical triangle (light yellow) is limited anteriorly by the median line (dashed line) of the neck, posteriorly by the anterior margin of sternocleidomastoid muscle, superiorly at its base by the inferior border of the mandible, and inferiorly at its apex by the manubrium. The posterior cervical triangle (orange) is limited anteriorly by the posterior border of sternocleidomastoid muscle, posteriorly by the anterior border of trapezius muscle, superiorly by the superior nuchal line of the occipital bone, and inferiorly by the middle third of the clavicle.
Imaging Modalities
Ultrasound (US) is usually the first line imaging examination for neck masses in children, being an excellent screening tool. Computed tomography (CT) uses ionizing radiation which is a major concern among paediatric population given the radiosensitivity of children. CT has some advantages over US and magnetic resonance imaging (MRI), since it provides a good delineation of anatomy, bone structures and airspaces, and has good accuracy in detecting calcifications and fat. MRI provides examinations with excellent soft tissue contrast, offering superior lesion characterization, good assessment of lesion extent and surrounding anatomic structures, without using ionizing radiation. So, MRI provides simultaneously better diagnostic and pre-treatment surgical planning information. However, it is expensive with long scan times, reason why it is often necessary to sedate younger patients.
Anterior Midline Lesions
Thyroglossal Duct Cyst
Thyroglossal duct cyst (TDC) is the most common congenital paediatric neck mass and the second most common benign neck mass after benign lymphadenomegaly.3,4 TDC may be found from the foramen cecum to the level of the thyroid gland, being that 20-65% occur in the infrahyoid neck.5 Suprahyoid and hyoid level TDCs are usually midline, whereas infrahyoid TDCs can be paramedian and be embedded in the strap muscles of the neck.6 Because of its origin TDC may have associated ectopic functional thyroid tissue, and occasionally this may be the only functioning thyroid tissue in the body.
TDC may lie dormant and asymptomatic for years, appearing as a painless midline mass, or it may present as a rapidly enlarging painful mass when superinfected, frequently after an upper respiratory infection. Most are clinically apparent before 10 years of age.7 Since ectopic thyroid tissue may be found within a TDC, thyroid pathologic conditions, such as goitre or malignancy can occur in a TDC in the adult life.8,9 Thus, surgical removal is indicated.
The diagnosis is usually clinical, based on clinical history and physical examination, since TDC elevates with tongue protrusion and moves with swallowing. However, imaging studies play an essential role to document the presence of the thyroid gland before surgical removal of TDC. Ultrasound is the best imaging modality for this, being able to accurately demonstrate the cyst, and allowing appropriated thyroid gland evaluation. Note that TDC contains proteinaceous material, so some internal echoes, septa and debris may be seen at US in the absence of infection.10,11Infection should be suspected if additional findings are present, such as thickened and irregular wall, increased peripheral vascularity and surrounding fat stranding.(Fig. 2)
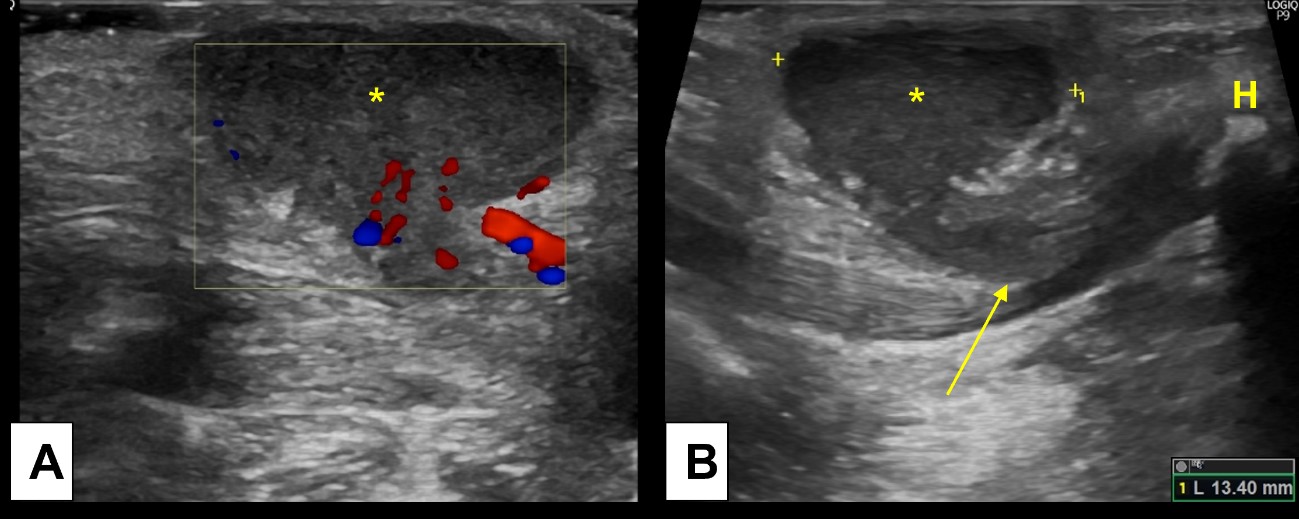
Figure 2: Inflamed thyroglossal duct cyst. A 2-year-old boy presents with a palpable midline tender nodule, clinically suspected of an inflamed TDC. Ultrasound [(A) and (B)] demonstrated an heterogenous midline cyst, with peripheral hypervascularity and oedematous changes of the surrounding fat, indicating inflammatory changes. (B) Note the linear path (arrow) connecting the TDC (*) to the hyoid bone (H).
Thyroid Nodules
Thyroid solid nodules can be benign or malignant. US is the imaging modality of choice for evaluation of the thyroid gland, giving important clues about the aetiology of the lesions. However, the definite diagnosis is made by fine-needle aspiration cytology. Cervical radiation therapy increases the incidence of both adenomas and thyroid cancer, which are otherwise rare in the paediatric population.10,11,12,13 Therefore, children with previous radiation need a long-term clinical follow-up and imaging the thyroid gland may be necessary.
Most of paediatric thyroid cancers are papillary carcinomas.14 Paediatric thyroid carcinomas are often diagnosed at an advanced stage, often presenting with cervical nodal and distant metastases (pulmonary and osseous).10,14 Yet, paediatric papillary thyroid carcinomas are usually well differentiated at histopathologic examination, having a very good prognosis, even when metastatic disease is present.(Fig. 3)14
Figure 3 Thyroid Carcinoma. A 15 year-old boy with a history of cervical radiation therapy for a posterior fossa ependymoma 4 years earlier, presents with a palpable thyroid nodule. Thyroid US (A) revealed a solid and hypoechoic nodule, with irregular borders, and microcalcifications at the transition isthmus/left lobe. Left cervical US evaluation (B) showed an enlarged ipsilateral cervical lymph node, with heterogenous ecostructure and microcalcifications, representing a metastatic cervical node.
Anterior Cervical Triangle Lesions
Branchial Cleft Cysts
Branchial cleft abnormalities constitute about 30% of congenital neck masses usually diagnosed in childhood or early adulthood, because of recurrent infection.17 Sinuses and fistulas are often diagnosed earlier than simple branchial cleft cysts (BCC), owing to external and/or internal mucosal drainage that increases the risk of superinfection.12 Radiological imaging studies are frequently used for the diagnosis, evaluation of its relations and to plan the surgical approach.
First BCCs are classically located in the anterior neck, between the external auditory canal, the mental symphysis, and the lateral aspect of the hyoid bone, or they can be intra-parotid. Second branchial anomalies may occur anywhere from the oropharyngeal tonsillar fossa to the supraclavicular region, most being in the submandibular space.18 Third and fourth branchial cleft anomalies are quite rare, occurring usually on the left side.1,19 These are anatomically very close, both extending from the piriform sinus, so radiological distinction may be difficult.19 Third BCC frequently lie in the posterior cervical space, posterior to the sternocleidomastoid muscle, being the second most common cystic lesion of the posterior cervical space after lymphatic malformations.20,21 Fourth BCCs are, in most cases, located adjacent to the thyroid gland, so they can present as suppurative thyroiditis in children.(Fig. 4)7,8,12,19
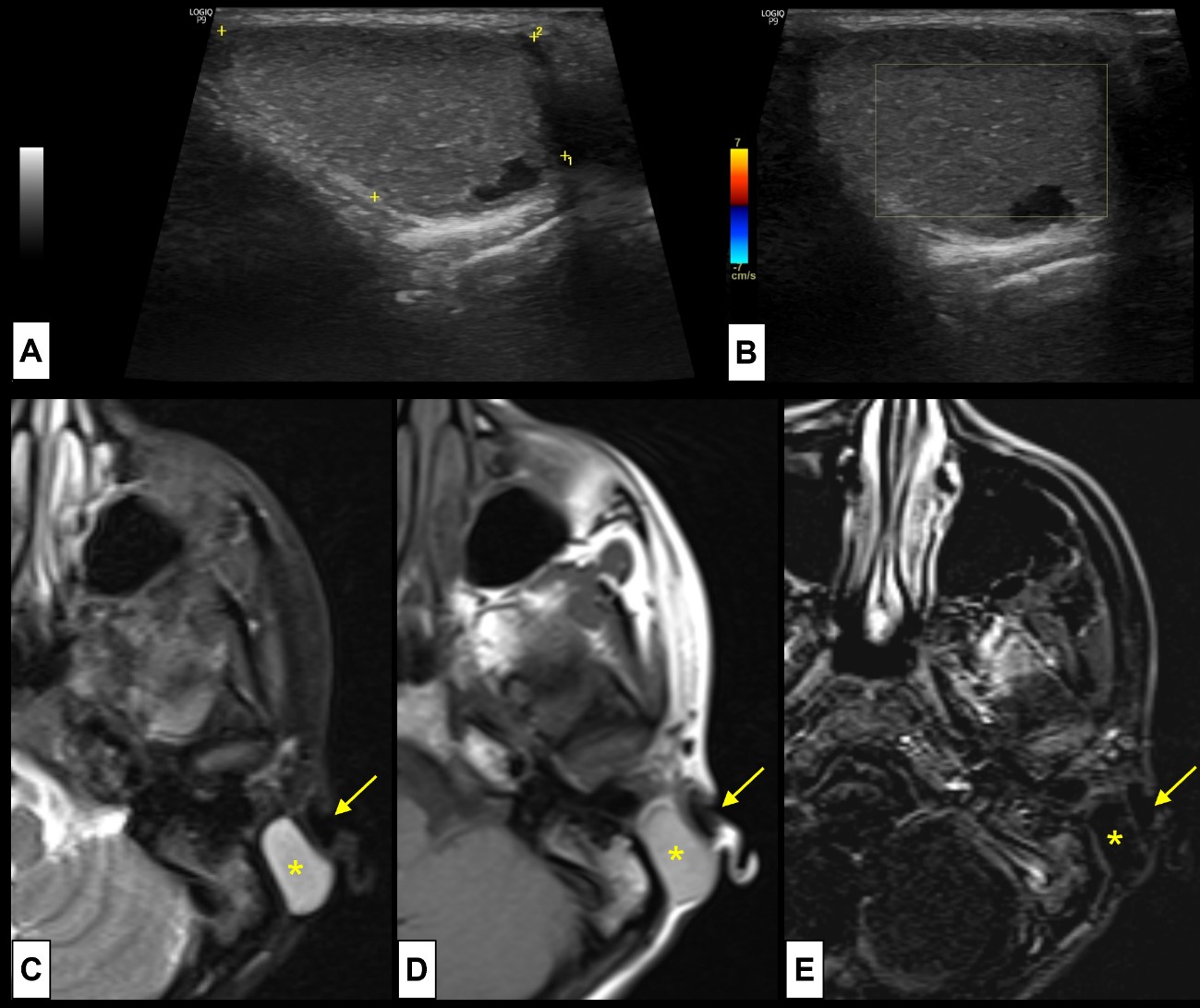
Figure 4: Branchial cleft cyst. A 15-year-old girl complains of recurrent episodes of a post-auricular painful tumefaction. US [(A) and (B)] demonstrated a post-auricular well defined cystic lesion, thin walled, with posterior acoustic enhancement and internal echoes, without (B) internal colour Doppler sign. No signs suggesting superimposed infection were present. It was thought to be a BCC, so for better characterization before surgery an MRI was performed. MRI [(C) axial T2WI-FS; (D) axial T1WI; (E) post-contrast T1WI-FS subtraction image] revealed a simple cystic lesion (*) in close relation with the external auditory canal (arrow). No sinus tracts were identified.
Ectopic Thymus
Thymus arises from the third pair of branchial pouches and descends caudally and medially on either side of the neck along the thymopharyngeal duct, adjacent to the carotid sheath, from the angle of the mandible to the thoracic inlet.8,15 These two embryologic anlages fuse at midline in the anterior mediastinum giving rise to normal thymus.15 Thymic tissue along this path should involute, if not, abnormally located thymic tissue develops, and thyropharyngeal duct cysts, intra-thyroid thymus or cervical thymus may occur.16
Cervical thymus corresponds to normal thymic tissue in an abnormal superior location at the neck. It is usually asymptomatic, diagnosed in the first decade of life as a painless midline or lateral neck mass, above the level of the brachiocephalic vein.12 As normal thymic tissue it usually involutes throughout life. US and MRI are the best imaging modalities for evaluation, revealing a mass with imaging characteristics like normal thymus.(Fig. 5)
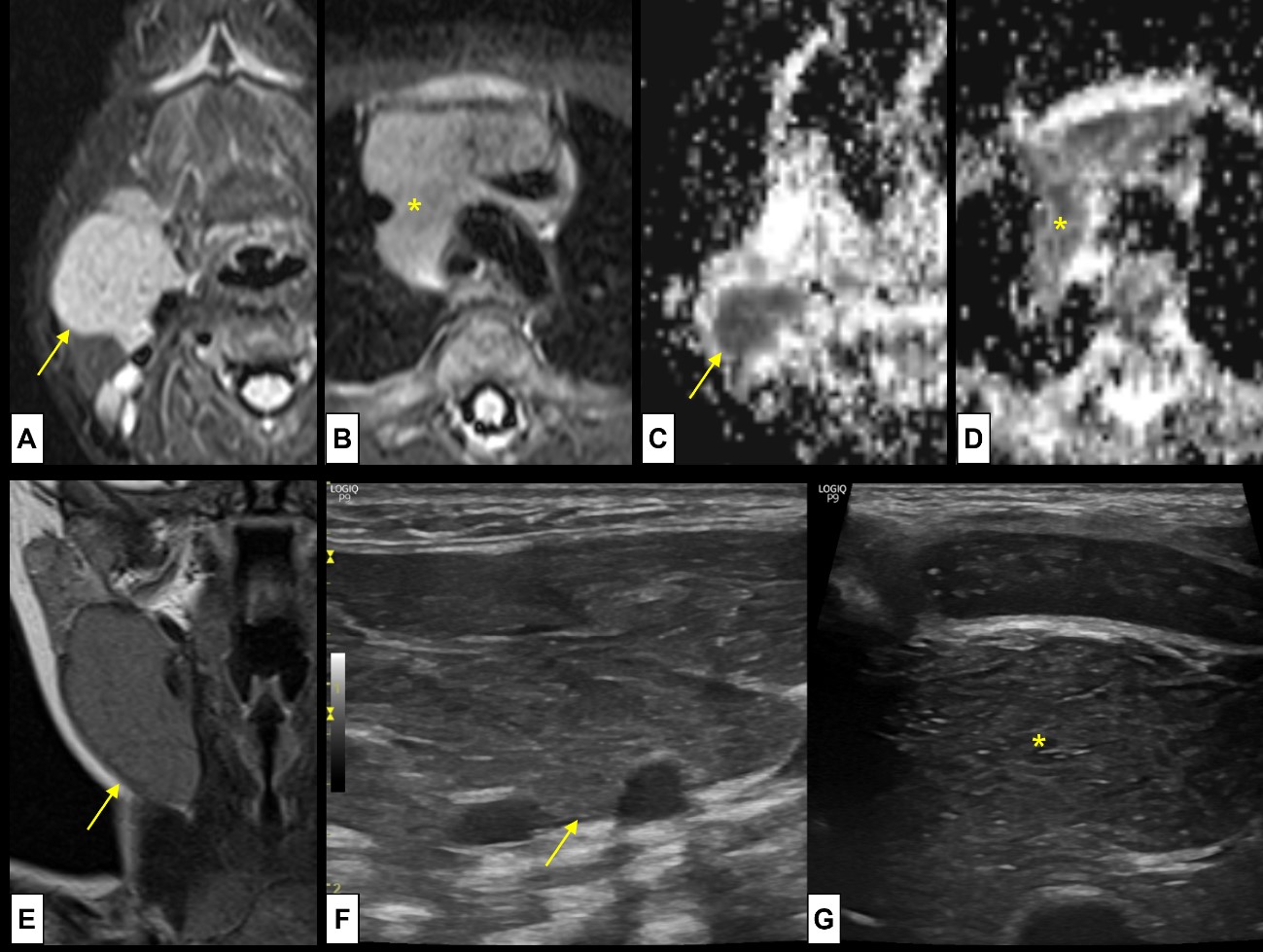
Figure 5: Cervical thymus. A 2-month-old boy was referred with a suspicion of vascular/lymphatic malformation, with a palpable soft mass at the anterior cervical triangle. MRI was performed [axial T2WI-FS (A) at the level of lesion and (B) at the level of the thymus; ADC map (C) at the level of lesion and (D) at the level of the thymus; (E) Coronal T2WI at the level of the lesion], showing (A-D) a mass (arrow) with the same signal pattern as the normal thymus (*), located (E) at the anterior cervical triangle, in the pathway of the thymopharyngeal duct. US was also performed [(F) cervical lesion and (G) normal thymus] showing the typical speckled appearance, with multiple linear hyperechoic septa and discrete homogeneously distributed hyperechoic foci at both locations. There was no continuity between the cervical thymus and the normal positioned thymus.
Lymphadenitis
The cervical region has a lot of lymph nodes grouped according to their anatomic locations, draining the head and neck regions. About 40% of infants have palpable cervical lymph nodes, and more than half are not pathologic.17 Yet, cervical lymphadenitis is by far the most common cause of neck masses in children.17
The cervical anterior triangle is the most frequently involved location, specially the submandibular nodes, since most lymphatics drain here.1,8,17 Viruses are the commonest agents and cause bilateral lymph node enlargement, frequently associated with mild, self-limited systemic symptoms. Bacterial infection more frequently causes acute unilateral lymph node enlargement, often associated with more prominent systemic manifestations.
Clinically, besides being palpable, inflamed lymph nodes are painful and can present with inflammatory skin signs, especially if from a bacterial infection. Lymphadenitis can present as isolated inflamed nodes or as adenopathic conglomerates. Complications of cervical lymphadenitis include suppuration and abscess formation.
Malignant lymphadenopathy manifests clinically as a painless, hard, and adherent nodal enlargement.22 It should be suspected when the lesion is present for more than 6 weeks, especially if the patient has risk factors for malignant node disease.
US is a great imaging tool for the evaluation of the cervical lymph nodes as it can distinguish simple lymphadenopathy from suppurative lymph nodes and a developing abscess.23 CT can assist in the evaluation of deeper cervical infections. In general, US is more specific, although less sensitive than contrast enhanced CT (CE-CT) for abscess detection.23
At US, normal lymph nodes are well defined ovoid structures, mostly hypoechoic, with a linear central hyperechoic/fatty hilum. During early childhood, the short axis of normal lymph nodes generally measures 5 mm or less.12 After the first decade of life it can measure up to 10 mm in the short axis.
Simple lymphadenitis is depicted as enlarged lymph nodes that may show increased vascularity at colour Doppler, maintaining their normal architecture.24 Incipient inflammatory changes of the surrounding soft tissues are usually present, appearing as hyperechogenicity of the fat planes. When suppuration occurs, the centre of the node becomes heterogeneously hypoechoic with loss of the hyperechoic fatty hilum, and as progressive necrosis develops, vascularity diminishes at colour Doppler. Central debris, septa, or foci of air may also be seen. (Fig. 6)
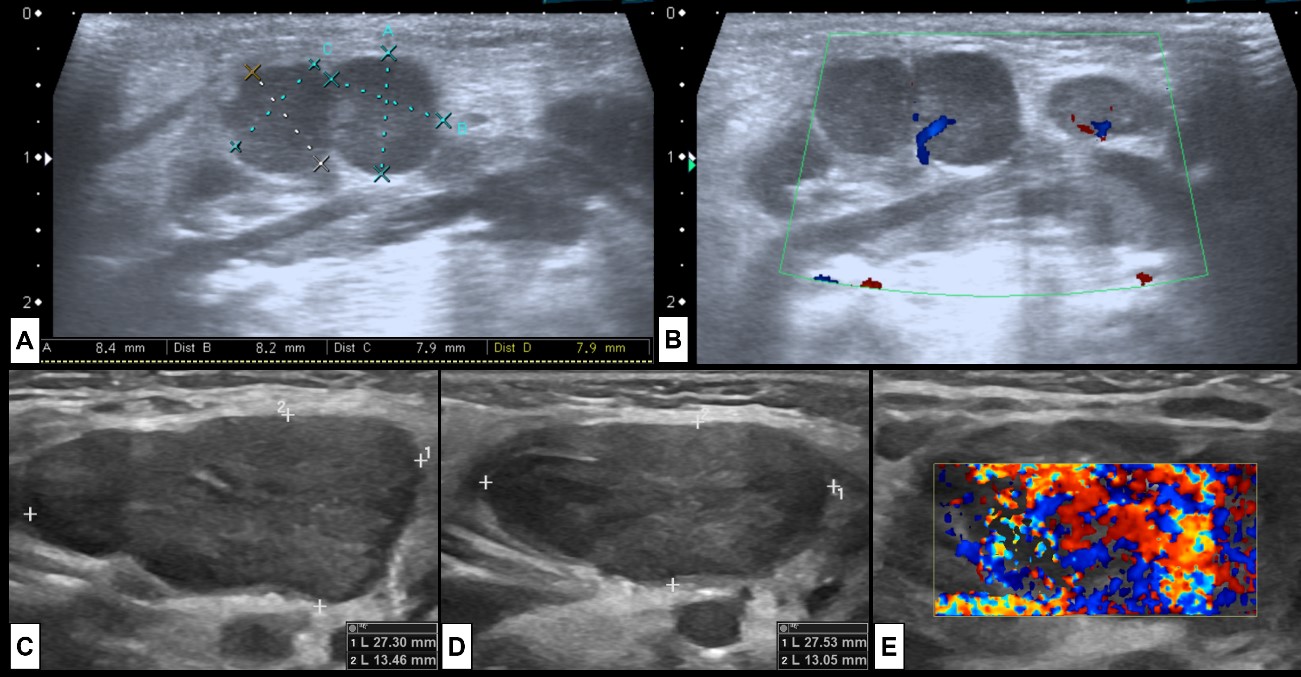
Figure 6: Adenitis. Two examples of cervical adenitis presenting as palpable masses. In the superior row the case of a 5-month-old boy that presents with submandibular tumefaction. US [(A) and (B)] was performed showing (A) some round prominent submandibular lymph nodes (level Ib), associated with hyperechogenic surrounding fat and (B) hypervascularization at colour Doppler, indicating inflammatory changes. In the inferior row the case of a 2-year-old girl with acute tonsillitis under antibiotherapy that develops new palpable cervical masses. US [(C) right side, (D) left side, (E) left side colour Doppler] shows enlarged bilateral level IIa lymph nodes, with associated inflammatory changes of the surrounding fat, and prominent vascularization at colour Doppler, specially at the left side. In both cases there are no signs of suppuration.
Fibromatosis Colli
In neonates or small infants, a palpable mass at the anterior cervical triangle is suggestive of fibromatosis colli (FC). FC is a benign fibrous proliferation within the sternocleidomastoid muscle, that manifests as fusiform enlargement of the muscle. It occurs more frequently in the lower third; however, it can cause diffuse enlargement of the muscle. It is believed to be the related with birth trauma with intramuscular haemorrhage that results in fibrosis, leading to muscle shortening.1,8,12,17
Clinically it usually presents as a firm growing neck mass, soon after birth (2 weeks), that eventually progresses with associated torticollis. The baby’s head tilts toward the side with the mass, and the chin tilts to the opposite uninvolved side. US is the first step in the workup, and characteristic imaging findings in conjunction with clinical features of congenital torticollis are usually diagnostic tools. Exceptionally, calcifications may be depicted as hyperechoic foci with acoustic shadowing.(Fig. 7)12
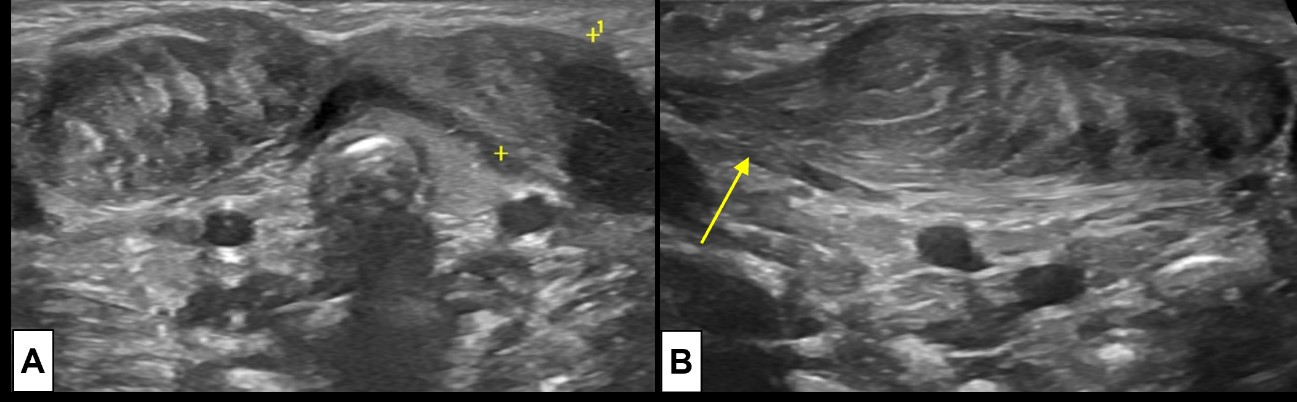
Figure 7: Bilateral fibromatosis coli. Requested US examination [(A) cervical midline transversal plane; (B) lateral right side longitudinal plane] for evaluation of a right cervical tumefaction in a 23 day old newborn demonstrates an ellipsoid region of muscle thickening at the lower half of both SCM muscles (A), the lesion is larger at the right side (B). It moved synchronously with the muscle, smoothly blending with the unaffected muscle fibers (arrow).
Mandibular Angle Lesions
Parotitis
When a lesion at the angle of the mandible is present, we should always bear in mind parotid gland pathology. A clinical evolution of less than 2 weeks indicates acute disease of inflammatory/infectious nature.
Acute parotitis is an infection of the parotid gland by a virus or, more rarely, a bacterial pathogen. Bacterial parotitis is most often unilateral, resulting from the spread of infection from adjacent structures or being associated with a parotid calculus.25
Acute suppurative parotitis is most frequent in children younger than 1 year or with systemic disease, frequently associated with a bacterial parotitis.8,11In the presence of parotid calculi, US may reveal dilated tubular structures, and a hyperechoic calculus causing acoustic shadowing may be seen.
Recurrent acute parotitis affects young children and resolves around puberty.26 It manifests as periodic episodes of unilateral or bilateral parotid gland swelling, with pain and fever.8,12 It has been associated with bacterial infections.(Figs. 8 and 9)
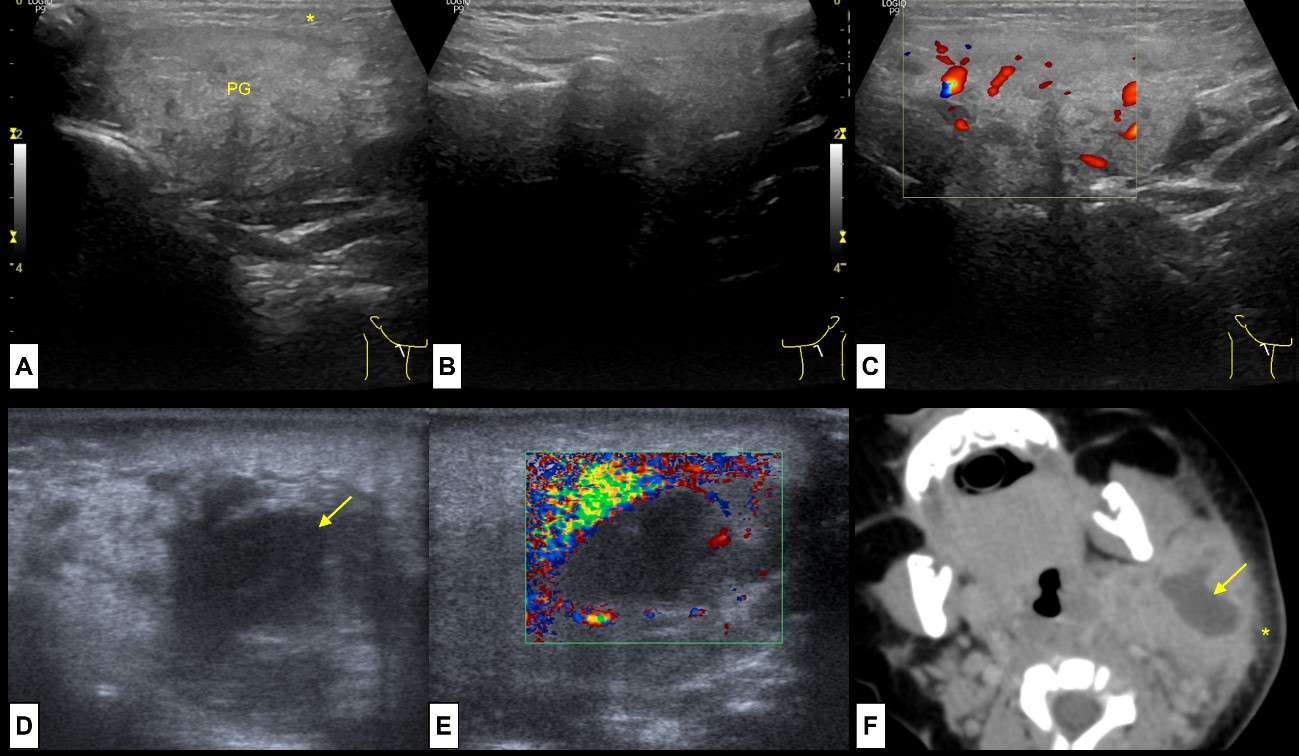
Figure 8: Acute and Suppurative Parotitis. A 11-year-old boy presents with pain, swelling and rubor at the right mandible angle. US revealed (A) enlargement of the right parotid gland (PG), slightly heterogeneous compared to the (B) left normal parotid gland. Note the oedematous changes on the adjacent subcutaneous soft tissue (*). On colour Doppler (C) the gland was hyperaemic. This represents acute parotitis of the right parotid gland. A 3 month-old baby girl presents with a left cervical tender mass. At US enlargement of the parotid gland was present, and (D) an ill-defined anechoic central region noted (arrow), without (E) colour Doppler signal, representing an intra-parotid abscess. CT (F) was performed to evaluate for deep extension, showing the enlarged parotid gland, with a central suppurative area (arrow) and oedematous changes of the subcutaneous soft tissues (*). These findings represent acute suppurative parotitis of the left parotid gland.
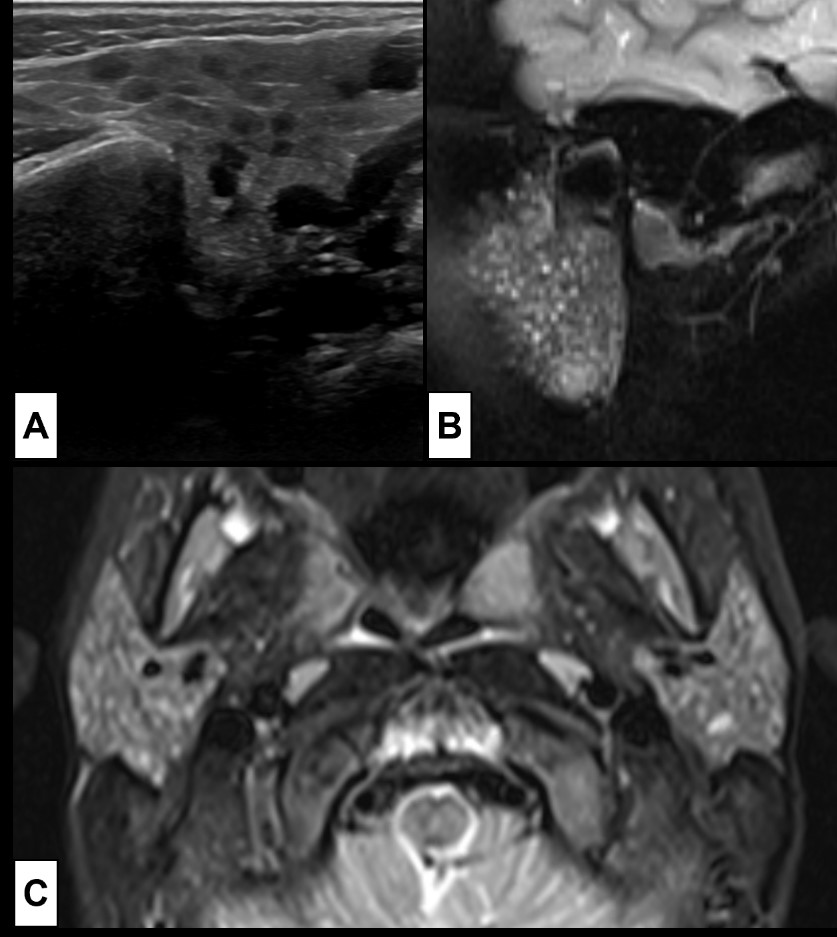
Figure 9: Recurrent acute parotitis. A 5-year-old boy with immunoglobulin A deficiency and a history of recurrent episodes of acute parotitis. At US (A), performed between acute episodes of parotitis, both parotid glands were enlarged, showing multiple millimetric hypoechoic foci. MRI [(B) coronal T2WI-FS and (C) axial T2WI-FS] was also performed, showing bilateral parotid enlargement with multiple tiny focus of high T2 signal, corresponding to the hypoechoic focus seen on US. These foci are likely to represent lymphocytic infiltration or sialectasis.
Parotid Infantile Haemangioma
Infantile haemangiomas (IH) are benign vascular neoplasms, composed of vascular channels lined by normal endothelial cells. Haemangiomas are the most common tumours of the head and neck in infancy, being also the most common tumour of the parotid gland in the paediatric age.23,27 IH are not present at birth, becoming apparent usually during the first month of life.
Haemangiomas are often diagnosed by history and physical examination. They typically display a rapid proliferation phase during the first year of life followed by an involution phase with gradual regression which can take up to 10 years.23,28 Clinically IH manifest as flat erythematous lesions or localized areas of telangiectasia. When IHs extend deeper, they may have a more bluish hue. During proliferation, haemangiomas may feel rubbery and warm, with a possible bruit.12,23 Imaging is reserved for questionable diagnosis, large lesions, and surgical planning.(Fig. 10)
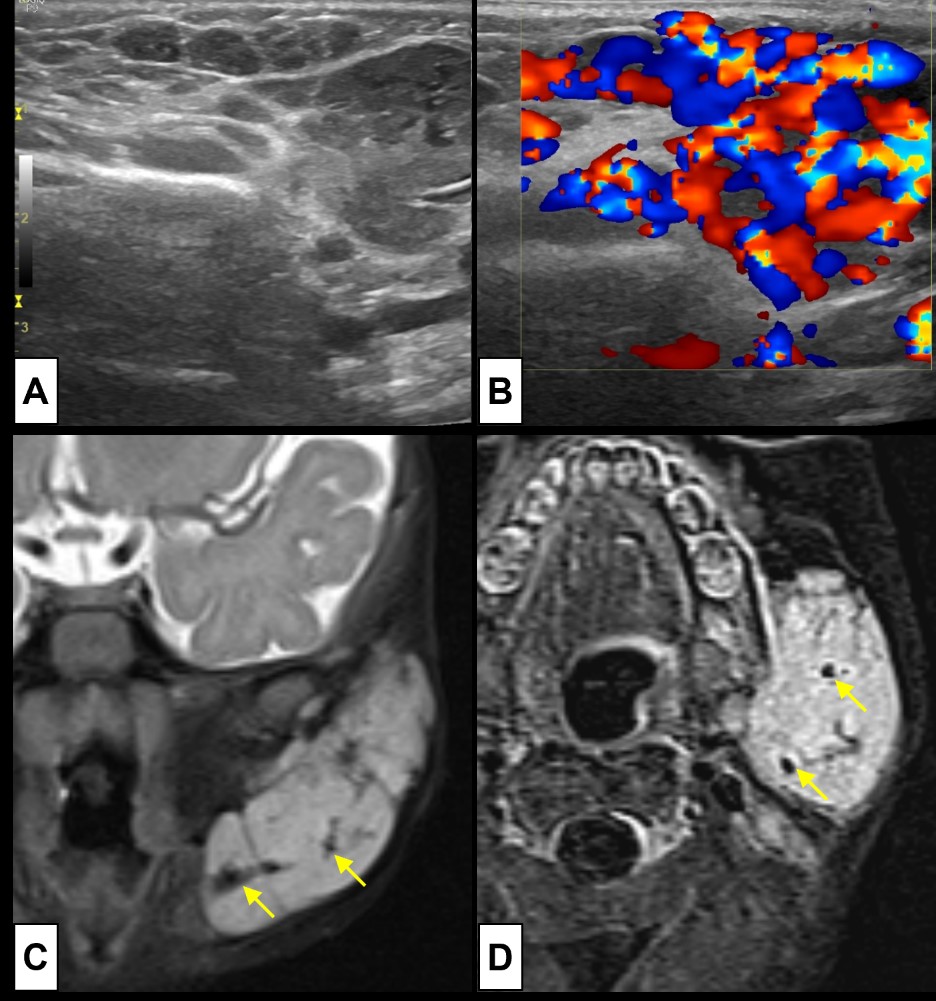
Figure 10: Parotid haemangioma. A 6-month-old girl presents with a rapidly enlarging, painless, soft mass at the angle of the left mandible. US [(A) B-mode; (B) colour Doppler] revealed a (A) heterogenous hypoechoic mass replacing the left parotid gland. On colour Doppler (B) imaging prominent internal arterial and venous vascularity was depicted. To better characterize and assess the extension of the lesion, MRI was performed [(C) coronal T2WI-FS; (D) axial post-contrast T1WI-FS subtraction image], revealing an (C) extensive lesion with high T2WI signal, with some internal thin septa and flow voids (arrows), that shows (D) prominent contrast enhancement according to its vascular nature.
Plunging Ranula
A simple ranula is a retention cyst of the sublingual gland, secondary to stenosis and/or occlusion of its duct.29 It contains high levels of salivary amylase and occurs in the mouth floor.17,29 Simple ranulas may expand and rupture, forming a pseudocyst that herniates through a mylohyoid muscle defect or around the posterior edge of the mylohyoid muscle, appearing as a lateral neck mass along the inferior border of the mandible.8,17 In this case the term plunging ranula is applicable. Ranulas are more frequent in adults, occurring very rarely in children.17 They are clinically evident at the mouth floor as a fluctuant swelling with a bluish translucent colour. Imaging evaluation is usually performed preoperatively to evaluate the sublingual gland, the extent of fluid extravasation, and delineate the defects through which the extravasation enters adjacent spaces. Transoral excision of the affected sublingual gland is the most common surgical treatment.(Fig. 11)17,29
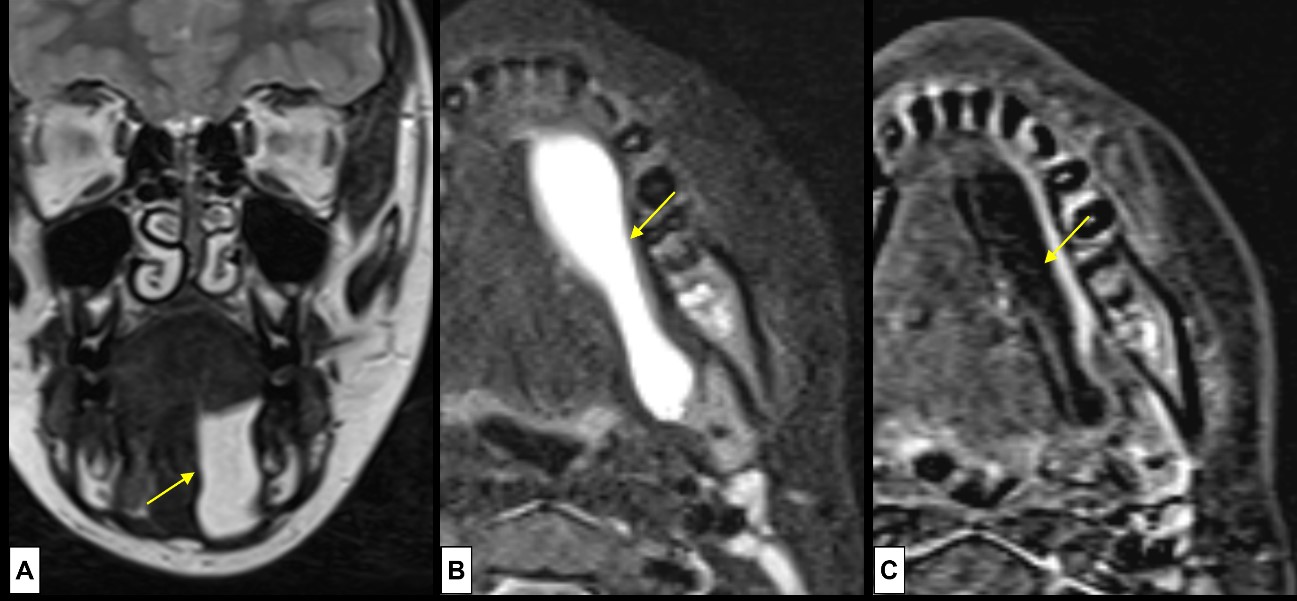
Figure 11: Plunging ranula. A 10-year-old boy complains of a small, soft, non-painful, nodule at the angle of the left mandible. US was inconclusive, so MRI [(A) coronal T2WI, (B) axial T2WI-FS, (C) post-contrast T1WI-FS subtraction image] was done, revealing a cystic mass (arrows) at the mouth floor, in the sublingual space, with posterior extension, “diving” into the submandibular space, below the mylohyoid muscle. The lesion demonstrates (A and B) simple fluid signal intensity at T2WI, and minimal peripheral enhancement (C).
Posterior Cervical Triangle Lesions
A palpable lesion at the posterior cervical triangle is most probably a vascular malformation. Vascular malformations are congenital benign anomalies that are present at birth and enlarge throughout life.12 There is a wide range of vascular malformations, being classified into five subgroups according to the primary cell makeup (ISSVA classification):30
Simple vascular malformations I: Capillary malformations (CM);
Simple vascular malformations IIa: Lymphatic malformations (LM);
Simple vascular malformations IIb: Primary lymphedema;
Simple vascular malformations III: Venous malformations (VM);
Simple vascular malformations IV: Arteriovenous malformations (AVM) and Arteriovenous fistula (AVF).
AVM and AVF are high-flow lesions. All the others are low-flow lesions, which are common in the neck.12
Lymphatic Malformations
Lymphatic malformations (LM), previously called lymphangiomas, are composed of abnormal lymphatic tissue. Most are detected by age of 2 years, constituting approximately 5% of all benign masses in infancy and childhood, frequently occurring in the head and neck region.31 In the suprahyoid neck they are more frequent in the masticator and submandibular spaces, and in the infrahyoid neck usually occur in the posterior cervical space.23 These can be classified according to the size of the lymphatic cavities as macrocystic (>1cm), microcystic (<1cm) or mixed cystic.30
LM are typically slow growing asymptomatic soft masses, although rapid volume increase may occur secondary to haemorrhage, trauma, or infection. Smaller lesions have been reported to regress spontaneously and can be clinically inapparent.1 Larger LM tend to occupy more than one space in the neck, frequently infiltrating adjacent structures or insinuating between them without significant mass effect.23
At US, LM appear as multilocular cystic masses with internal septa of variable thickness. Occasionally solid components may be present.12 Microcystic LM may appear as a hyperechoic lesion because of numerous interfaces, giving a spurious solid appearance. The cystic components are frequently anechoic, unless they have a high lipid content, internal haemorrhage, or infection, in which case increased echogenicity or fluid-fluid levels can be identified.12,32 Doppler US imaging may detect flow in the septa.23
Since LM can become very large lesions, CT and MRI are useful for delineation of anatomic location and extent of disease in relation to the surrounding vital structures. MRI has the best spatial resolution for accurate delineation of LM margins. On MRI LMs are readily diagnosed, appearing as well-circumscribed lesions that frequently involve more than one cervical space, with internal cystic spaces separated by thin enhancing septations. Superimposed infection alters the enhancement characteristics. Microcystic LMs may be more confusing because tiny cystic spaces may simulate a diffusely enhancing lesion.(Fig. 12)
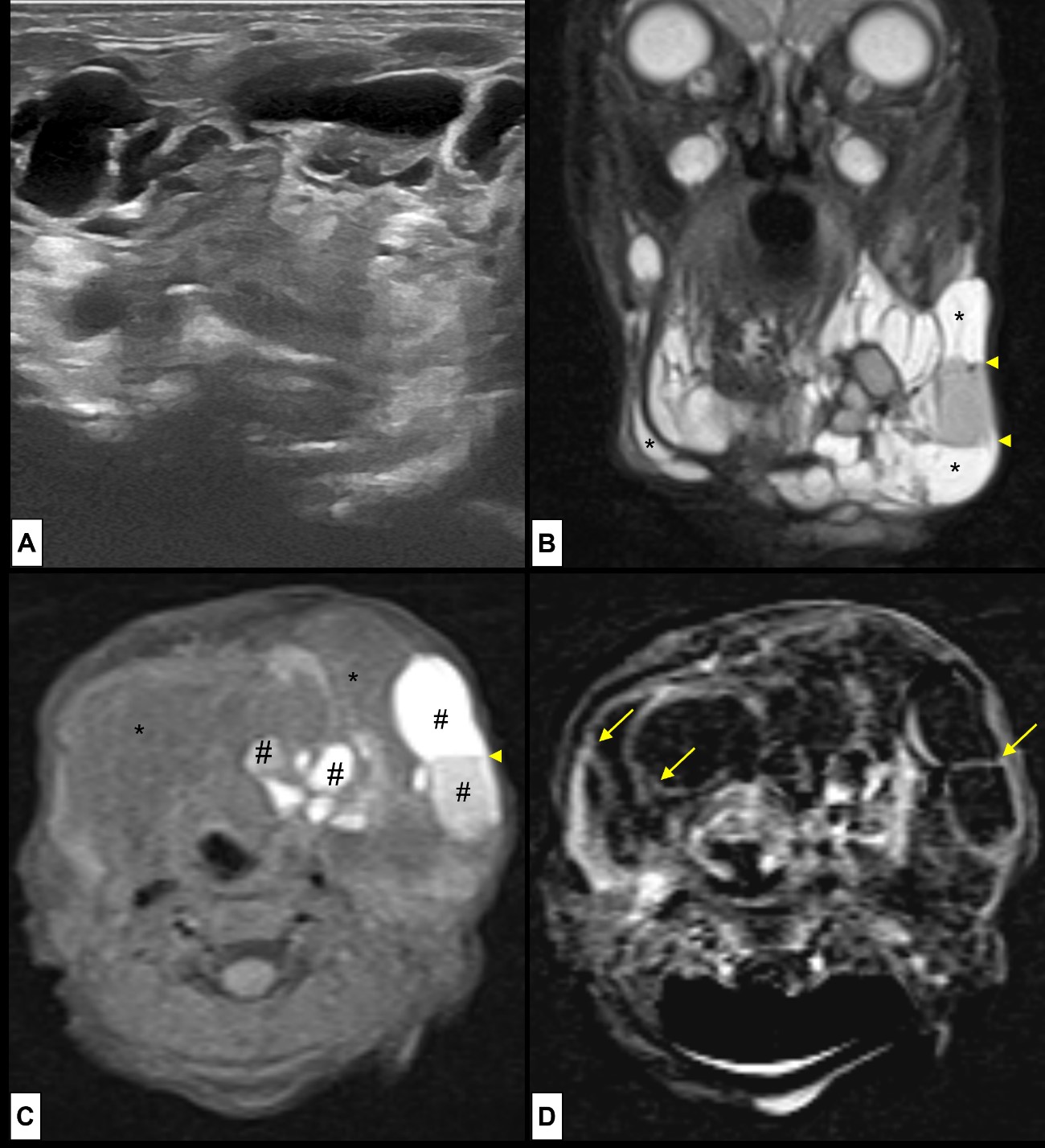
Figure 12: Lymphatic Malformations. A new-born girl presents in the first day of life with a left sublingual tumefaction, and two days later the tumefaction progresses becoming a large left cervical mass. At clinical inspection it was a cystic, clear fluid, painless lesion. Ultrasound (A) was performed revealing a multiloculate large cystic lesion. MRI [(B) coronal T2WI-FS; (C) axial T1WI-FS; (D) post-contrast T1WI-FS subtraction image] was performed for accurate delineation of LM margins. On MRI the lesion was well-circumscribed, composed of multiple cystic spaces (*) hyperintense at (B) T2WI and hypointense at (C) T1WI. The cystic spaces were large, typical of a macrocystic LM. Some fluid-fluid levels are depicted (arrowheads), within locules of variable high T1WI signal (#), revealing the presence of haemorrhagic/high protein content. Post contrast image (D) only shows enhancement of the thin septa (arrows). Vascular flow voids are absent.
Either Cervical Triangle Lesions
Abscess / Deep Neck Infection
Neck abscesses frequently develop from suppurative lymphadenitis, so these are very frequent in the parapharyngeal and retropharyngeal spaces. However, the most frequent neck abscesses in children are the peritonsillar ones, secondary to acute tonsillitis.5 Deep neck infections with abscess formation have significant potential morbidity and may lead to upper airway obstruction.
US has a limited accuracy in detecting deep neck infections, due to the limited field of view in deeper structures. The skill level of the operator or the noncooperative young child can also interfere with ultrasound results.17 At US we can see a heterogeneously hypoechoic or anechoic mass with a variable thickness, irregular and hyperaemic wall of solid tissue. Adjacent lymphadenopathies may be identified. CE-CT is highly sensitive for the detection of deep neck infections, giving accurate information about the location, extension, and anatomic relations of the abscess. However, it is not very specific since accurate distinction between cellulitis, phlegmon and abscess can be hard.23 The presence of lymphadenitis causes significant cellulitis, which presents as diffuse low attenuation within the soft tissues and muscular planes due to oedema, with obliteration of normal fat planes, without rim enhancement. A focal hypodense collection without rim enhancement or with an incomplete rim of enhancement is indicative of phlegmon. Abscesses appear as hypodense collections with a complete rim of enhancement. (Fig. 13)
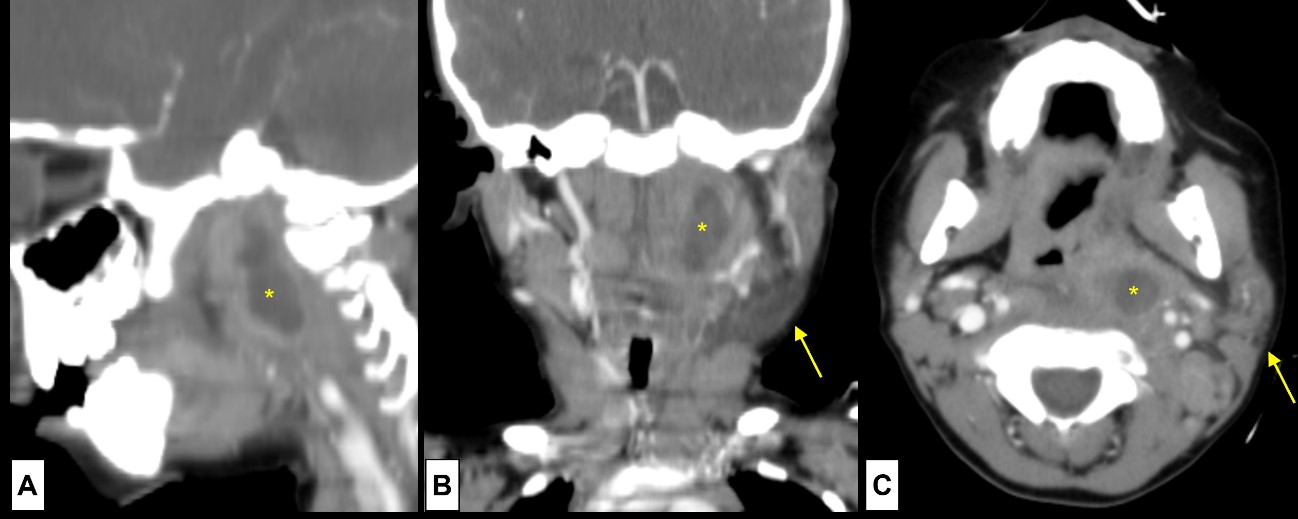
Figure 13: Tonsillar abscess. A 3-year-old boy presents with acute tonsillitis. Three days after starting oral antibiotics clinical worsening occurred, and a left cervical tumefaction was noted. At this point, a deep neck abscess was suspected, so CE-CT was performed [(A) sagittal plane; (B) coronal plane; (C) axial plane]. The diagnosis of a tonsillar abscess was confirmed by noting the presence of a circumscribed fluid collection (*) at the left tonsillar bed. Note the contour asymmetry of the neck (arrow), clinically seen as a left cervical tumefaction.
Rhabdomyosarcoma
Rhabdomyosarcoma is a malignant tumour that arises from skeletal muscle cells. It is the most common soft tissue sarcoma in children and the second most frequent malignancy in the paediatric head and neck region.13,33 Histologically it can be divided into three main subtypes: embryonal, alveolar and pleomorphic.13 Embryonal rhabdomyosarcoma is the most common subtype overall and among children.13
Rhabdomyosarcoma imaging appearance is non-specific and tissue sampling is required for a final diagnosis.34 However, imaging is required to assess local extent and stage the disease, and to assess therapeutic response. CE-CT and MRI are the best imaging modalities for this. Nevertheless, US is useful in the evaluation of more superficial rhabdomyosarcomas and in the identification of suspicious adjacent lymph nodes.34
CT usually demonstrates an inhomogeneous soft-tissue mass, spontaneously isodense to muscle.5,23 Rhabdomyosarcomas frequently cause bone erosion, which is best visualized with CT, so the skull base should be carefully evaluated for erosion or foraminal widening.
MRI is especially useful in assessing perineural spread along cranial nerves and intracranial extension. Postcontrast images are very helpful for detection of metastases and accurate delineation of the tumour extent.(Fig. 14)
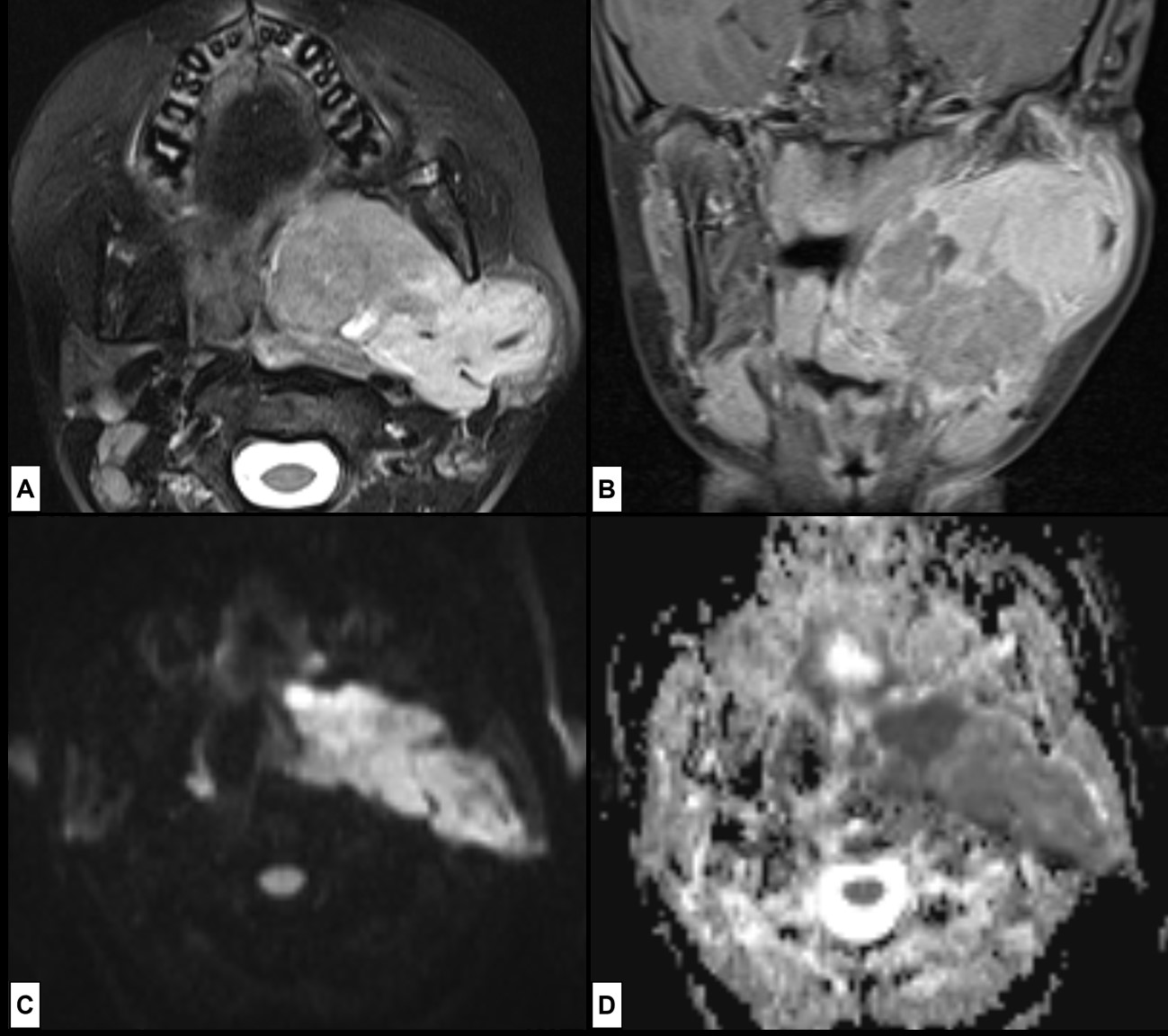
Figure 14: Embryonal Rhabdomyosarcoma. A 10-year-old boy presents with a rapidly enlarging mass at the angle of the left mandible noted two weeks before. At clinical evaluation a large parapharyngeal lesion was seen. MRI [(A) axial T2WI-FS; (B) coronal post-contrast T1WI-FS; (C) axial DWI at b1000; (D) axial ADC map] was done to characterize the lesion. (A) A large, well-circumscribed soft tissue mass, slightly inhomogeneous, mostly T2 hyperintense compared to muscle, was seen. Inhomogeneity is seen because of frequent intratumoural haemorrhage and necrosis. At post-contrast images (B) intense enhancement of the lesion is seen, and better delineation of its limits possible. The lesion shows intense restricted diffusion (C and D).
Osteosarcoma
Osteosarcomas are malignant tumours of the connective tissue that primarily produce osteoid matrix, with variable amounts of cartilage matrix and fibrous tissue.35 Osteosarcoma is the most common primary malignant bone tumour affecting children and adolescents. Currently numerous types of osteosarcomas are listed, being the most common the conventional subtype, which is a high-grade intramedullary osteosarcoma.36 These are aggressive lesions with rapid doubling times and therefore often large at the diagnosis. Conventional osteosarcoma most frequently affects long bones, unusually involving the facial bones.36
Clinical manifestations are nonspecific, with pain and swelling being the most frequent symptoms.36 Treatment of conventional osteosarcoma consists of chemotherapy, followed by wide surgical resection.
Conventional osteosarcoma are most frequently mixed sclerotic and lytic lesions, with variable amounts of cloudlike opacities within the lesion, that represent osteoid matrix production.36 They tend to disrupt the cortex, without causing bone expansion, which is associated with aggressive periosteal reaction and soft-tissue masses.36 Radiographic features of conventional osteosarcoma are characteristic, so the radiographic diagnosis is usually straightforward. However, for lesions in complex anatomic areas or of small size, cross-sectional imaging may be required.36
At CT, the osteoid matrix components are hyperdense, like normal bone density. The nonmineralized portions demonstrate soft tissue attenuation and are seen replacing the normal, low attenuation, bone marrow. Chondroblastic components have higher water content, demonstrating lower attenuation. Compared to MRI, CT is better to visualize osteoid matrix, so it can better detect small foci of calcification in a predominantly lytic lesion.
MRI is preferred in the preoperative evaluation, since its greater soft tissue contrast allows better assessment of extraosseous extent of the lesion, which is critical in planning surgical excision.36 Osteosarcomas present as masses of intermediate signal on T1WI, with areas of hyperintensity replacing the normal marrow on T2WI. Mineralized matrix presents as areas of low signal on both T1WI and T2WI. Foci of central haemorrhage and necrosis are common.(Fig. 15)
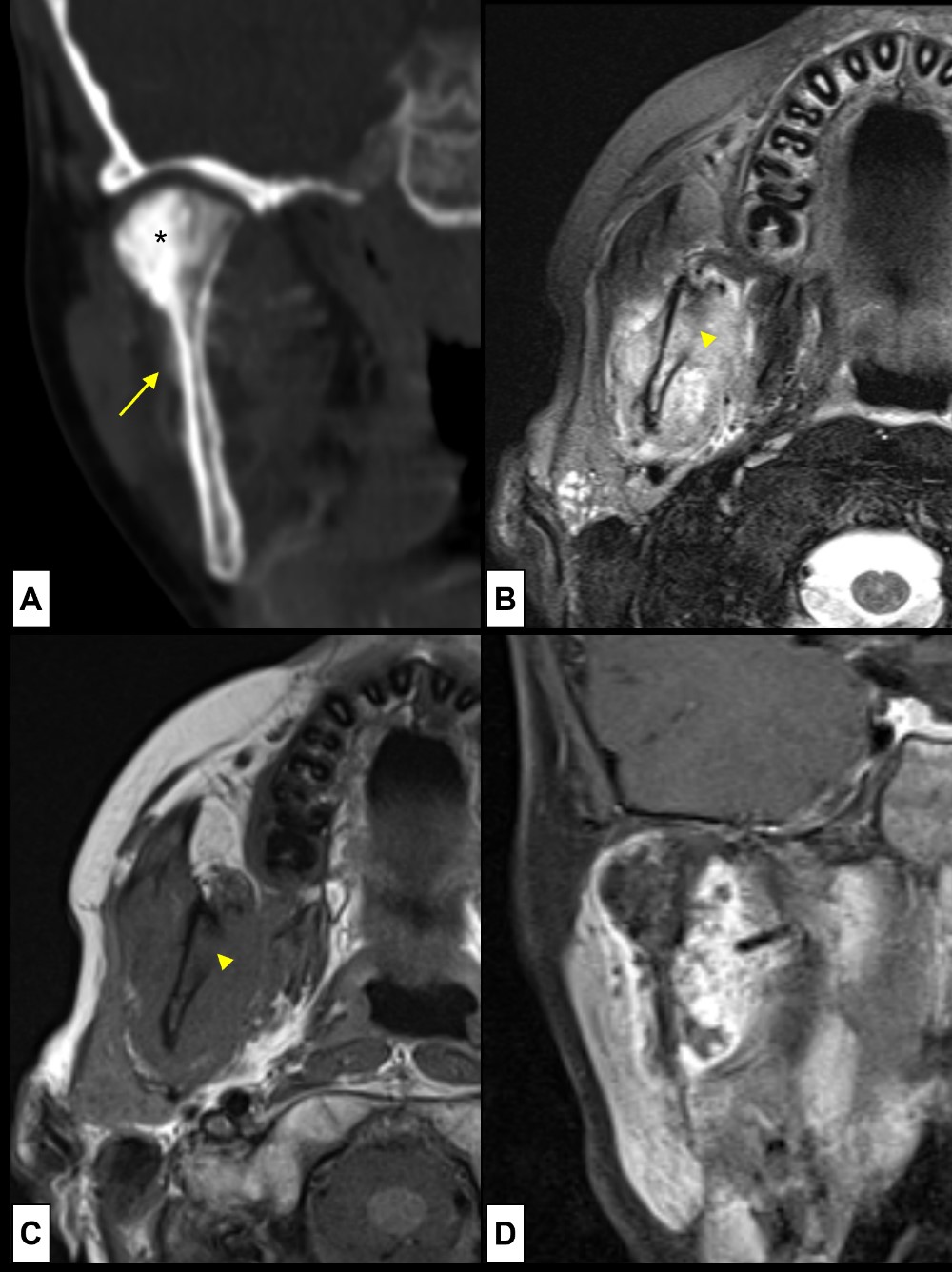
Figure 15: Osteosarcoma. 12-year-old boy with mandibular pain for one week. At physical examination a right temporal and mandible angle tumefaction is evident. (A) CT (coronal bone window) revealed expansion and sclerosis of the mandible condyle (*) and ramus, along with aggressive pattern of periosteal reaction (arrow). Soft tissue component was also noted so MRI was performed [(B) axial T2WI-FS; (C) axial T1WI; (D) coronal post-contrast T1WI-FS]. At MRI the soft tissue mass had heterogenous T2WI signal (B), involving the masticatory muscles and masseter, disrupting the cortical bone (arrowhead). The soft tissue lesion is (C) homogenously hypointense at T1WI, showing (D) intense enhancement after contrast administration, with some non-enhancing internal areas, corresponding to necrotic components. The findings are of an aggressive lesion, of osseous origin, so biopsy was suggested, revealing an osteosarcoma.
Lipoblastoma
Lipoblastoma is a rare benign tumour occurring exclusively in childhood.32 Two forms have been described: the circumscribed lipoblastomas are encapsulated, superficial lesions; Lipoblastomatosis are deep, poorly circumscribed lesions with an infiltrative growth pattern.32,37 Most lipoblastomas arise in the extremities and trunk, with only a few occurring in the neck.32
Histologically lipoblastomas are mainly composed of mature fat cells deposited in variable amounts of myxoid stroma with a prominent plexiform capillary network.37,38 Lipoblastomas usually present as a painless, rapidly growing, soft mass or swelling.37 The main differential diagnoses are dermoid cyst, hibernoma and myxoid liposarcoma.32,37 However, liposarcoma is exceedingly rare in the paediatric population. Complete surgical removal is usually the first-line treatment for lipoblastomas and imaging follow-up recommended for at least 5 years.37
On US lipoblastoma appears as a well-defined hyperechoic mass with internal septation. If the myxoid components predominate it will be mostly hypoechoic.37 At CT lipoblastomas are heterogeneous lobulated masses containing non-enhancing regions of fat attenuation with intervening soft-tissue enhancing septa.37,39 MRI is the best imaging technique for the accurate diagnosis of lipoblastoma. It shows as a solid, encapsulated and lobulated mass with a signal intensity that follows that of fat in all sequences, exhibiting marked decreased signal at fat suppression sequences.37 Due to the presence of myxoid material, the T1WI signal is usually less intense than that of fat. Lesions with a predominant myxoid component may not display the usual macroscopic fat signal. In these cases, gradient echo sequences allow the detection of microscopic fat in the lesion.(Fig. 16)
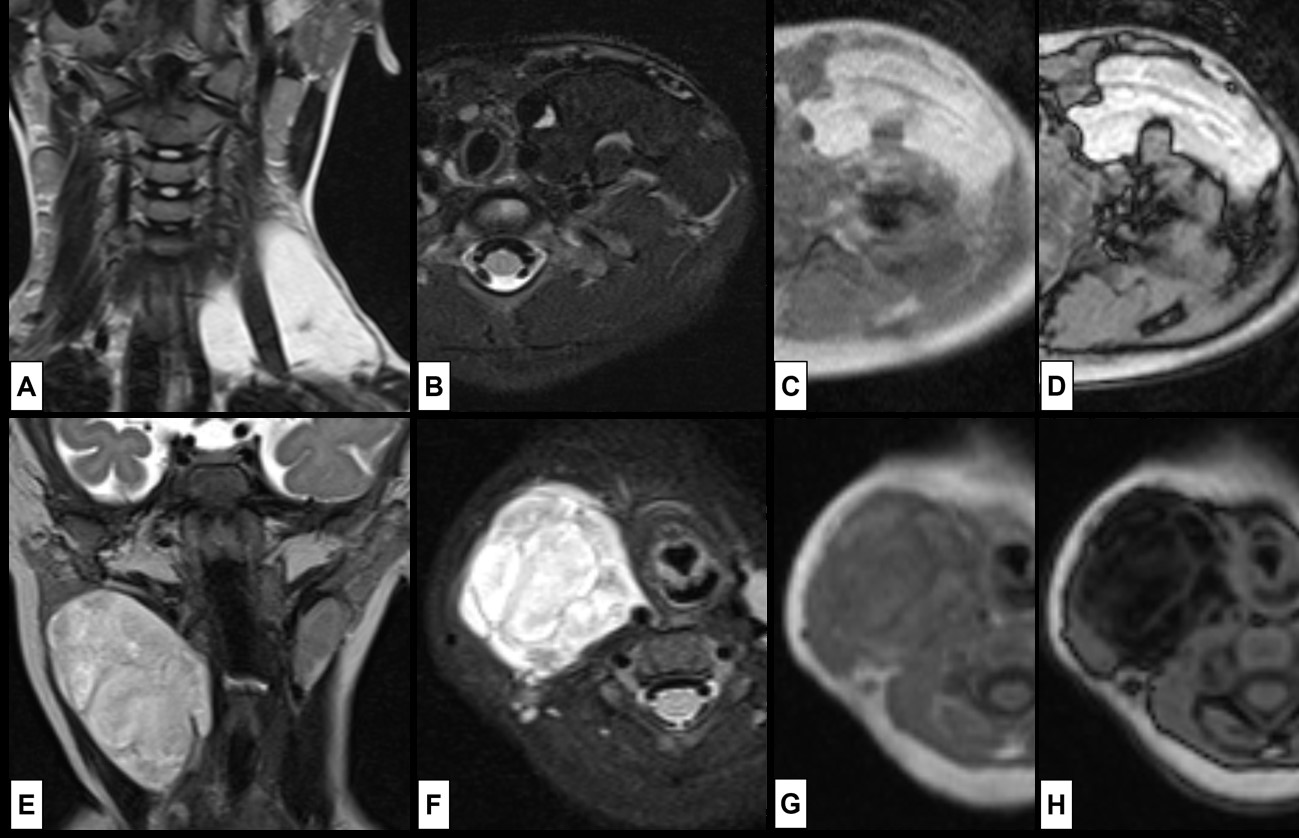
Figure 16: Lipoblastoma. In the superior row the case of a 4-year-old girl that presents with a painless left supra-clavicular mass, noted some months before. MRI [(A) coronal T2WI; (B) axial T2WI-FS; (C) T1WI in-phase; (D) T1WI out-phase] shows a (A) hyperintense T2WI lesion surrounding the scalene muscles, composed of mature adipose tissue, which explains the loss of signal at (B), without signal drop at (D), yet India ink artifact is seen at the margins of the lesion, indicating the presence of fat-water interface. In the inferior row the case of a 6-month-old girl that presents with a painless right cervical mass, noted 2 weeks before. MRI [(E) coronal T2WI; (F) axial T2WI-FS; (G) T1WI in-phase; (H) T1WI out-phase] revealed a (E) slightly heterogenous hyperintense T2WI lesion. However, the lesion exhibits high signal at (F) T2WI-FS, which suggest the presence of myxoid components. The signal drop seen in (H) compared to (G) reveals the presence of microscopic fat in the lesion as well.
Neurofibroma
Neurofibromas are benign tumours arising from the nerve sheath and along with schwannomas are the most common neurogenic tumors.8,12 They can be sporadic, but, especially in children, they are frequently associated with neurofibromatosis type 1 (NF1).8 This autosomal dominant phacomatosis is characterized by the development of widespread neurofibromas, which may be solitary or plexiform. Solitary neurofibromas grow along the path of the nerve, appearing as a slow growing uniform, elongated masses.8,12 Plexiform neurofibromas appear as multiple multilobulated masses or fusiform enlargement of peripheral nerves.23,32 It usually involves a long segment of a major nerve trunk and its branches, producing a bag-of-worms appearance.32 These can extend into adjacent structures such as skin, muscle, bone, and internal organs. Neurofibroma malignant degeneration can occur, particularly in patients with NF1, originating malignant peripheral nerve sheath tumor (MPNST).32,40 MPNST are difficult to distinguish from neurofibroma at imaging, its development is suggested by a rapid increase in size, change in enhancement characteristics or the development of metastatic disease.12,40
Children with known NF1 affecting the cervical region may develop airway obstruction caused by impingement of multiple or large tumours.1 Since there is no curative treatment, surveillance and selective tumour excision or debulking is the mainstay of management.1 Accurate surveillance may be accomplished with MRI evaluation to detect deeper, non-palpable, lesions.
On US neurofibromas simulate cysts, appearing as well circumscribed hypoechoic lesions, with posterior acoustic enhancement.12,23 It may also show a target appearance of peripherally hypoechogenicity and central hyperechogenicity.41 On CT neurofibromas are generally slightly hypoattenuating compared to muscle.23 At MRI neurofibromas appear as T1WI hypointense and T2WI hyperintense lesions, showing variable contrast enhancement.8 On T2WI the target sign may be present, manifested by a central hypointensity against a background of hyperintensity.23 The target sign can help distinguish plexiform neurofibromas from lymphatic malformations, which tend to involve multiple fascial compartments as well.(Fig. 17)40
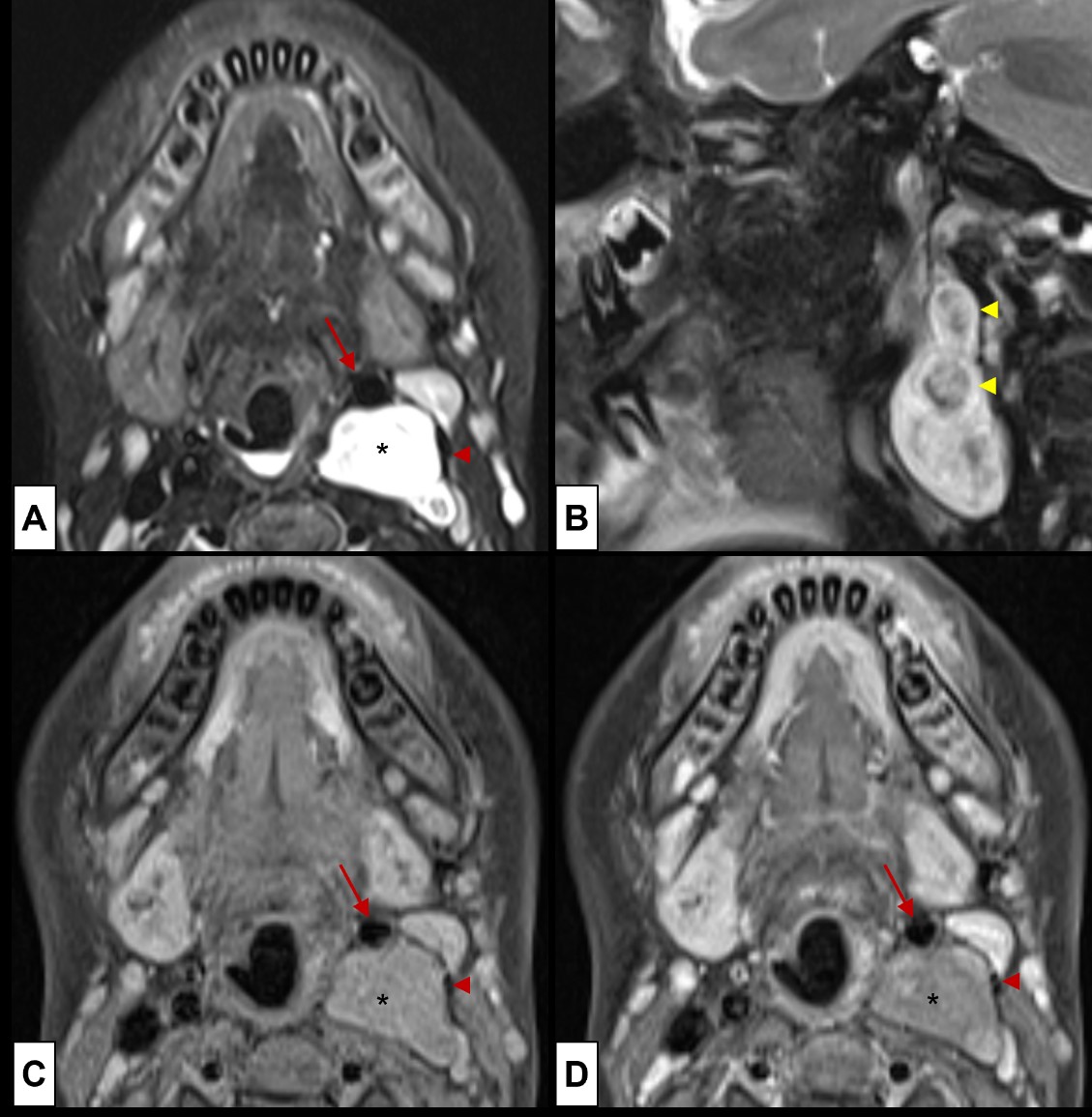
Figure 17: Neurofibroma. A soft palpable left cervical mass is noted at the routine physical examination on a 7-year-old boy with known NF1. It was suspected of a nerve sheath tumour, and MRI [(A) Axial T2WI-FS; (B) Sagittal T2WI-FS; (C) axial T1WI-FS; (D) axial post-contrast T1WI-FS] was done for evaluation. A mass in the left carotid space was depicted, showing (A) high T2WI signal (*), with (B) an elongated morphology and exhibiting the target sign (yellow arrowhead). It has (C) low T1WI signal, (D) slightly enhancing in a heterogenous way. The mass displaces the common carotid artery anteromedially (red arrow) and the internal jugular vein posterolaterally (red arrowheads), witch points in favour of lesion of the vagus nerve.
Conclusion
Paediatric neck masses can have many different aetiologies. We present a review of some of the most common and other less common pathologies that can present as cervical masses in children. By discussing the location and clinical history of the different pathologies, we can reduce the differential diagnosis list. This approach helps the radiologist to better define not only the most appropriate imaging modality to correctly assess the patient, but also to achieve the most probable diagnosis more confidently.