Introduction
Coronaviruses have been identified as human pathogens since 1960.1 On the 31st December 2019, a cluster of pneumonia cases of unknown cause detected in Wuhan, China, was first reported to the World Health Organization (WHO)2 and led to the discovery of a new coronavirus which was named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and the disease it causes, coronavirus disease of 2019 (COVID-19).1-2 SARS-CoV-2 rapidly spread across several countries, being declared by WHO as a public health emergency of international concern on the 30th January 2020 and as a pandemic on the 11th March.2
Although around 81% of patients develop mild symptoms,3 clinicians should be aware of the possibility for rapid deterioration one week after symptom onset,3-4 with development of severe illness, namely acute respiratory distress syndrome (ARDS). Despite this being the major complication described in COVID-19 cases, others may arise, involving the cardiovascular, renal and neurological systems.1,4 It has been proposed that the severity of SARS-CoV-2 infection in some cases may be explained by a state of hypercoagulability with the development of thrombosis in various organs.1,3-4
Therefore, it is mandatory to be aware of a possible coagulopathy onset, especially in high-risk patients. On that account, primary care professionals, as front-line providers of healthcare in COVID-19 mild cases, have a privileged position for monitoring the illness’ evolution, warning signs and prevent negative outcomes.
In this review the aim is to perform a synthesis of the published scientific information, to characterize coagulation disorders in adult patients with SARS-CoV-2 infection and to determine both their pathophysiology and the best diagnostic procedures to use. We also intend to analyze the response to anticoagulant or antiplatelet therapy in adult COVID-19 patients, to conclude on the utility of these agents either in a prophylactic or therapeutic use, both in hospital and ambulatory settings. Since this is an emerging field, with complex and heterogeneous evidence, the most adequate kind of study to be performed seems to be a scoping review. By using a more expansive inclusion criteria, it allows to adequately summarize research findings and identify research gaps in the existing literature.
Methods
Overview
A systematic search was performed to identify scientific literature on coagulation disorders in adults infected with SARS-CoV-2. The present scoping review was conducted according to both Joanna Briggs Institute Guidelines on Scoping Reviews5 and Preferred Reporting Items for Systematic Reviews and Meta-Analyses Scoping Review guidelines (PRISMA-ScR).6 The protocol of this scoping review was previously registered on the Open Science Framework (OSF) website (osf.io/zm843).
Search strategy and selection criteria
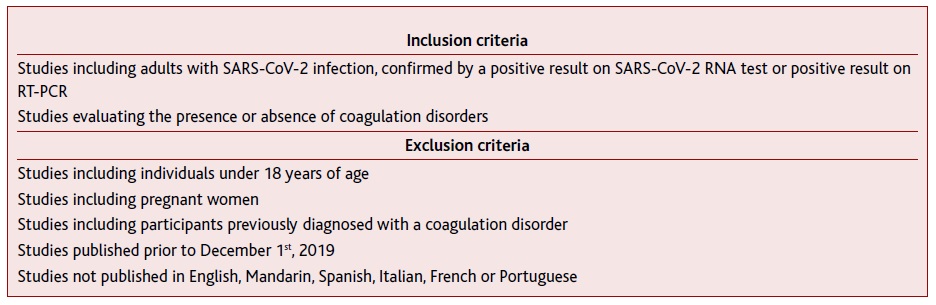
A search on the MEDLINE®, SciELO® and Web of Science® databases was performed between the 1st and 2nd May 2020. Different combinations of the Medical Subject Headings (MeSH) words, full entries and variations were essayed but, because of the novelty of the topic, we have chosen not to use MeSH terms (Appendix 1. Search queries according to database). The criteria for studies’ eligibility shown in Table I were applied.
The search included articles published between the 1st of December 2019 and the 2nd of May 2020. The initial date was established to limit the search to articles regarding the new coronavirus and to account for any reports of the infection. Considering that the topic in this study is very recent and a shortage of robust evidence is expected, no restrictions were applied based on study design and both peer-reviewed and non-peer-reviewed material were included. No exclusion according to country or healthcare level was implemented and no additional review of the selected articles’ references was performed.
Selection process
For minimizing the risk of selection bias, the query was performed systematically in three different databases, strictly applying the mentioned methodology. No restrictions were applied based on the availability of abstract or study type. All retrieved articles were combined on Mendeley® software, and duplicated articles were excluded.
The articles selection was divided into three sequential stages: based on title, on abstract, and on full article reading. At each stage, articles were assessed against the inclusion and exclusion criteria mentioned above and accepted or rejected accordingly.
Researchers were differently paired for the selection of articles by title, abstract, and full article reading. The method of confrontation and consensus was used and when consensus could not be reached within each group, the final decision was taken by the whole research team by a simple majority.
Data extraction and charting
A team of two researchers extracted data independently and discrepancies were resolved using the method of confrontation and consensus, after a whole team briefing to uniformize criteria. Each article was characterized, when applicable, according to authors, publication date, publishing institution, country of origin, article type, aim/purpose of the selected study, study population, sample size, methodology, intervention type and duration, comparator, outcome measures, key findings that relate to the scoping review question, study limitations, and level of evidence according to SORT classification (Appendix 2. SORT taxonomy).7 Based on our main objective, the extracted data were organized into each of the following categories: characterization of coagulation disorders, pathophysiology, laboratory diagnosis, imaging diagnosis, and treatment.
Results
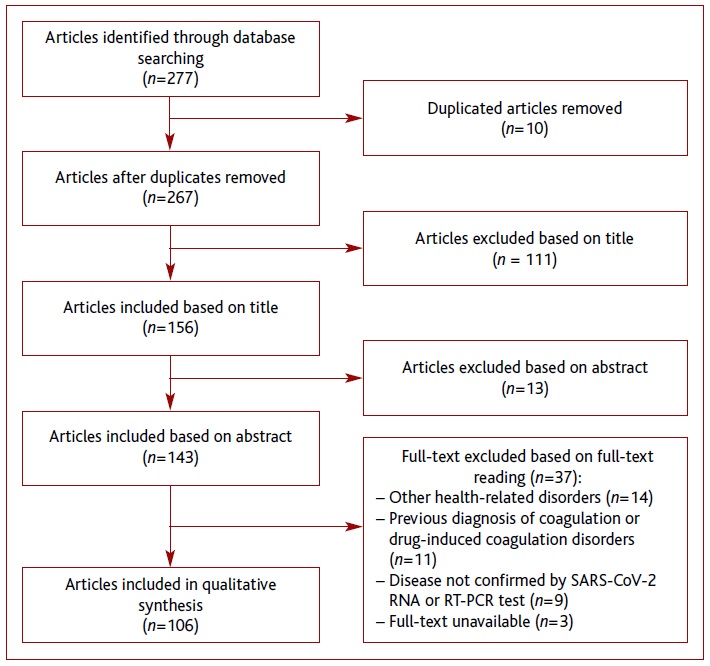
After the literature search, a total of 277 records were obtained, of which 10 were duplicated. During the selection process, 161 articles were excluded, resulting in 106 for qualitative synthesis. A literature search is shown in Figure 1.
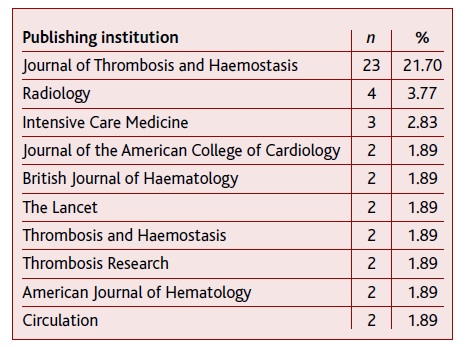
The included articles were published in 63 different institutions, the top three ones being Journal of Thrombosis and Haemostasis (n=23), Radiology (n=4), and Intensive Care Medicine (n=3). Table 2 shows the top 10 institutions with publications included in this scoping review.
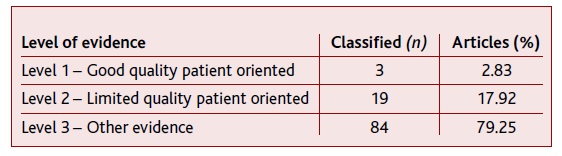
China (n=31), USA (n=18) and Italy (n=15) were the three out of 18 countries with most articles published. As far as the article type is concerned, letters complied 33.97% (n=36) of the sample, in which eight (7.55%) were preliminary studies. Still, 26.42% (n=28) corresponded to original studies, 23.58% (n=25) to reviews and 13.21% (n=14) to case reports; metanalysis (n=1), commentary (n=1) and consensus papers (n=1) were also included. In total, 21.70% (n=23) of the articles had been submitted to the peer-review process. The distribution of classified articles according to the level of evidence is shown in Table 3.
As for the main topics addressed, 55 articles concerned ‘characterization of coagulation disorders’ and 30 articles referred to ‘pathophysiology’. ‘Laboratory diagnosis’ was analyzed in 46 articles and ‘imaging diagnosis’ in 17 articles. Regarding ‘treatment’, it was mentioned in 56 articles, with anticoagulation therapies, antiplatelet agents, and other therapies being addressed in 42, five, and nine articles, respectively. Still, no articles about anticoagulant or antiplatelet prophylactic or therapeutic use in COVID-19 patients in ambulatory setting was found, which limits one of the aims of our scoping review.
Finally, at the time of the elaboration of the data chart, no articles were found about diagnostic tools, nor about therapeutic or prophylactic strategies for coagulation disorders in out-patients followed mostly at a primary care setting.
Summary of evidence
The relevant evidence found is summarized in “Supplementary Material ” (https://www.rpmgf.pt/ficheiros/supplementary_material_supplementary_table_1.pdf) and is classified according to provenance.
Conclusions
Critical appraisal
Viral infections can affect all stages of the coagulation process, mainly by overactivation of the coagulation cascade. This mechanism aims to limit the spread of the pathogen as part of the host defense system, potentially leading to syndromes such as disseminated intravascular coagulation (DIC) in severe cases. Accordingly, respiratory tract infections have been reported to increase the risk of both deep venous thrombosis and pulmonary embolism (PE).8-10
a. Characterization of coagulation disorders
Particularly for coronavirus infections, it seems that dysregulation of the coagulation cascade and the subsequent formation of intra-alveolar or systemic fibrin clots are prominent findings.11 As a matter of fact, COVID-19 has been associated with thrombotic and hematologic complications, as once observed for severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1) and Middle East respiratory syndrome coronavirus (MERS-CoV) infections.12 For instance, thrombocytopenia was identified in 17 over 47 laboratory-confirmed MERS-CoV cases in Saudi Arabia13 and a retrospective analysis of 157 SARS-CoV-1 infected patients in a Hong Kong hospital revealed the presence of thrombocytopenia in 55% of the cases.11
There is still no consensual data on the burden of thromboembolic complications in COVID-19 patients. Different frequency rates both between studies and in different kinds of thromboembolic events were found. It has been reported overall venous thromboembolism (VTE) frequency of approximately 20% and an incidence of stroke of approximately 3%, which are notoriously higher comparing to other acute respiratory infections such as pH1N1 influenza, which has an estimated prevalence of thromboembolic events of 5.9%.14 In the light of these results, it seems that thromboembolic phenomena are especially frequent in patients with more severe forms of COVID-19, resulting in a frequency variability according to the place of observation and treatment (general ward versus intensive care unit (ICU)). Although the real prevalence and incidence of VTE in the ICU remain unclear, it is in this setting that higher rates are found, probably because patients having thromboembolic phenomena are often admitted to the ICU.15 Thus, caution is needed when making inferences regarding patients’ risk of thromboembolism in this context. Furthermore, there is no information regarding the incidence or prevalence of thrombosis ei-ther after hospital discharge or in patients treated at home. Therefore, it was not possible to determine the global impact of coagulation issues yet and this remains a focal point for future research.16
b. Pathophysiology
The intricate relationship between the triad consisting of inflammation, coagulation changes, and endo-thelium dysfunction seems to be the basis for an explanation of the pathophysiological changes underlying thrombotic phenomena in COVID-19 patients.
The activation of the inflammatory response by different mechanisms, but mainly mediated by cytokines, will cause damage and abnormal activation of the coagulation system, pathologically manifested as a generalized small vessel vasculitis and extensive microthrombosis.17-19 Since inflammation is one source of the problem, it seems important to explore the role of anti-inflammatory drugs in the treatment of COVID-19.20-21
Regarding the coagulation pathways, the included articles showed that viral infections seem to be able to cause an imbalance between procoagulant and anticoagulant mechanisms, leading to a state of hypercoagulability. The increase of thrombin22 also seems to play an important role, by further enhancing inflammation via proteinase-activated receptors (PARs). Moreover, increased plasmin levels are also implicated, by promoting adhesive fibrin formation and deposition, in addition to massive platelet activation and consumption.
According to the obtained data, hypercoagulability is still favored by a pro-adhesive and pro-coagulant dysfunctional endothelium, due to direct damage23 and inflammation-mediated injury.19 However, these findings, in line with more recent studies,24 state that there is still not enough evidence indicating an intrinsic procoagulant effect of SARS-CoV-2.
Overall, although the prevalence of clinical thrombotic events in COVID-19 patients warrants further studying, it may be determined by multiple factors, namely infection severity, the patient’s prior condition, and therapeutic/prophylactic strategies9 that will probably become available soon and may be based on the pathophysiology, in particular, the three main disease mechanisms mentioned above.
c. Laboratory diagnosis
Although it can be potentially dangerous to solely use laboratory biomarkers to guide clinical decisions regarding which patients can be discharged and which ones need admission,25 it seems important to establish more cost-effective and rapid screening methods for COVID-19-associated coagulopathy. Clinical manifestations of thrombotic phenomena in COVID-19 patients may remain unnoticed due to their overall severity. Hence, monitoring of certain thrombosis-associated parameters seems mandatory in patients with sudden onset of hypoxia, respiratory distress, hemoptysis, signs of heart strain, or reduced blood pressure.26-27 The analyzed data, corroborated by more recent studies,24 state that a rising pattern in D-dimer, prothrombin time (PT), and activated partial thromboplastin time (aPTT) and a decrease in fibrinogen and platelet count after admission are signs of DIC evolution and, thus, are determinant for an early clinical approach.
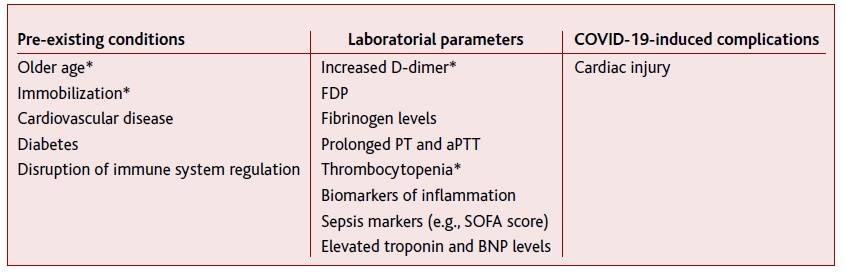
Furthermore, the information found indicates that all hypercoagulability-related phenomena may be aggravated in the presence of comorbidities, such as diabetes28 and obesity.29-30 Effectively, when trying to predict the COVID-19 evolution, several factors have been associated with mortality and poor outcomes (Table 4).
Laboratory parameters shown in Table 4 seem to be effective prognosis biomarkers, in particular, increased D-dimer levels and thrombocytopenia, and should integrate the routine assessment of all hospitalized COVID-19 patients. However, further studies are required, mainly to identify adequate cut-off values and to establish assessment frequency.
The creation of predictive severity scores that gather all the above parameters would also be of a major utility. Several authors recommend different scores that may be relevant for an early suspicion of coagulation disorders, such as the International Society on Thrombosis and Haemostasis (ISTH) score for DIC and the ISTH SIC score for sepsis-induced coagulopathy (SIC). Yet, although the ISTH score for DIC demonstrated to be a useful adverse prognostic marker,31 the use of the ISTH SIC score poses some problems. In a study in Tongji Hospital of Huazhong, from 449 patients with severe COVID-19, only 21.6% met the SIC criteria with the use of the SIC score.32 Until now, this was the only study to evaluate this screening tool in COVID-19 patients.17,29,33 In fact, SIC criteria seem to require further adjustments to correlate better to COVID-19 severity since the sole determination of D-dimer six times the upper limit of normal did comprise a higher proportion of severe cases (161 of 446; 35.9%).31 Furthermore, these scores have not been applied to the outpatient setting or mild cases of SARS-CoV-2 infection yet. Therefore, in line with a more recent study,24 included articles support that there is no robust evidence on how and when to use these tools and, above all, on their validity and security when making clinical decisions, warranting properly conducted prospective studies.
d. Imaging diagnosis
Imaging techniques, including lung and vascular ultrasound and chest computed tomography (CT), have been employed to diagnose coagulation phenomena. The existing studies enrolled a small number of patients and the role of integrated use of these techniques is still not clear. It seems important to determine when to perform a CT scan in COVID-19 patients to rule out PE. Al-though evidence advocates its use whenever the patient’s clinical state suffers deterioration, there is not enough data to answer this question in other situations. Moreover, a CT scan may not always be feasible34 and raises the concern about ionizing radiation. Thus, a lung ultrasound screening could play an interesting role, given its several advantages (absence of radiation, lower cost, and viability for repetition). However, it has also important limitations, namely its lower sensitivity in comparison to chest CT scan, given that it cannot usually detect deep lesions in pulmonary parenchyma,35 and that it generally requires experienced users. Again, outpatients were not addressed in studies regarding the use of lung ultrasound, which is another significant limitation.
e. Treatment
Given the increased thromboembolic risk of COVID-19 patients, analyzed data show that early introduction of anticoagulation could theoretically be beneficial to decrease the risk of major organ damages. In this sense, some authors advocate that thromboprophylaxis with low molecular weight heparin (LMWH) during the earlier phases of SARS-CoV-2 infection may have a positive effect, not only in terms of thrombosis prevention but also by decreasing systemic and pulmonary inflammation and limiting viral invasion.36 However, there is not enough data to support the use of a ‘universal’ thromboprophylaxis strategy, as the use of anticoagulants seems not to benefit unselected COVID-19 patients.32 In fact, in the ambulatory setting, routine thromboprophylaxis is not recommended, even for those with acute medical illness or respiratory symptoms. Thus, further investigation is needed to clarify the real utility of thromboprophylaxis on disease outcomes and prognosis in outpatients.
On the other hand, given the higher incidence of VTE in hospitalized COVID-19 patients, particularly in those with severe illness, it has been strongly advised that all inpatients should be considered for thrombopro-phylaxis with either LMWH, unfractionated heparin (UFH), or fondaparinux unless there are absolute contraindications.22,37-45 Furthermore, anticoagulation appears to be associated with lower mortality in the subpopulation meeting SIC criteria46 or with markedly elevated D-dimer levels.46-47 From the analyzed data, there were no bleeding complications reported, which seem to reinforce its safety.
As far as the type of anticoagulant drug is concerned, parenteral ones seem to be preferred. Moreover, a better prognosis is associated with anticoagulation with heparin, mainly LMWH, in patients having severe COVID-19.48-49 Although there are very little data on the use of direct oral anticoagulants (DOACs), they seem to have serious disadvantages in this context, due to potential drug-drug interactions with certain COVID-19 therapies.39,50 The lack of reversal agents or specific antidotes in most of the cases, further places these drugs in a high-risk category and strongly discourages their use in this setting.
The choice of the parenteral drug and dosage depends on the location and severity of the clot.17 Despite some unconformity in the analyzed data, the use of LMWH may have further advantages over UFH, due to lack of routine monitoring, less frequent blood draws, less heparin-induced thrombocytopenia, and familiarity among doctors.51 However, randomized clinical trials comparing both the efficacy and safety of LMWH versus UFH are required.
As far as dosage is concerned, there is no consensus on adequate LMWH prophylactic doses and some authors recommend its use according to the thrombotic risk of each patient.52 The optimal intensity of anticoagulant thromboprophylaxis requires further study. There are two different perspectives: some authors advocate that, in any hospitalized patient, COVID-19 associated coagulopathy should be managed as other disorders with similar characteristics,53 while others argue in favor of a ‘stepped up’ or intermediate-dose regimen. The latter uses a risk-adapted approach for escalating the dose of anticoagulation,54 probably based on the fact that some critically ill COVID-19 patients have increased VTE incidence even when using standard VTE prophylaxis.51,55-57 Interestingly, similar conclusions were reached in a study where H1N1-associated ARDS patients who received therapeutic anticoagulation with UFH or LMWH had 33-fold fewer VTE events than those treated with prophylactic doses of these drugs.58 Therefore, even though the present data do not support the prophylactic use of anticoagulant doses used in a therapeutic setting, there is an urgent need for randomized clinical trials comparing the efficacy and safety of higher doses of LMWH or UFH to those used prophylactically in COVID-19 patients.
Regarding the duration of thromboprophylaxis, including the post-discharge period, there are very little data available on this research. It has been suggested that it should be started during the pre-hospital phase, in case of pre-existing or persistent VTE risk factors. It is recommended to be administered for the entire duration of the hospital stay and to be maintained for at least 7-14 days after discharge.39,45 Nevertheless, its extended use should be considered in high-risk for VTE in patients, including those with pneumonia, sepsis, or any condition requiring management in an ICU setting, who are at risk of hospital-associated thromboembolism.59 Thus, as no robust evidence is available on the subject, an individualized patient-based approach is recommended, aimed at balancing the risk/benefit ratio and taking into consideration the underlying VTE risk factors, until proper clinical trials are performed.
As for therapeutic anticoagulation for COVID-19 patients, when indicated, the full dose should be given.37,45 Same as for non-COVID patients, therapeutic anticoagulation should still be considered if there is high clinical suspicion of VTE29 but imaging is not feasible,37 always taking into account the bleeding risk.29,37 When used therapeutically, LMWH dosage should be adjusted according to the patient’s renal function, age, and hemorrhagic risk.60-61
As indicated by the scarce existing data, antiplatelet agents have not been used as first-line choices either in prophylaxis or therapy, but they may play a role in SARS-CoV-2 infection. Antiplatelet agents62-63 such as clopidogrel,62 associated or not to heparin,63 can be useful in patients with thrombocytosis. Also, dipyridamole, because of its antiviral and antioxidant properties, seems to have beneficial effects in patients with COVID-19.39 However, additional research is needed to clarify the role of antiplatelet agents and the situations in which they should be used.
Several other agents and therapies have been used to prevent and treat coagulation disorders in COVID-19 patients, with different results. Regardless, insufficient evidence is available on the efficacy and safety of these drugs in patients with SARS-CoV-2 infection and, consequently, they cannot be considered yet.
Limitations
In this scoping review, an extensive, systematic, and robust search focused on the study objective was applied. Aiming to minimize the risk of selection bias, a systematic query comprising three different databases was performed, researchers were cross paired for the different steps of article selection, and a method of confrontation and consensus was used to solve divergencies.
Given the lack of robust evidence and the novelty of this scoping topic, a broad selection approach was chosen as far as article languages are concerned. Apart from publications in English, others in Mandarin, Spanish, Italian, French, and Portuguese were also included, some of which correspond to languages spoken in the most affected countries at the beginning of the COVID-19 pandemic and the first ones to publish data. Also, no restrictions were applied regarding the type of articles included. Letters were included as well, despite their frequent low level of evidence, since some of them describe preliminary studies that could have crucial information on the topic. Another reason for including letters was that, in a moment when all the existing information may be essential for establishing the more correct approach to the pandemic, authors and/or editors may have preferred to publish certain articles as letters, to obtain a more rapid publication. Fur-thermore, despite being aware of the importance of peer-review to the quality of articles and conclusions presented, both peer-reviewed and non-peer-reviewed material were included so that no information would be missed. Once more, during the pandemic, because of the need for rapid information transmission, many journals decided to publish articles with non-peer-reviewed material. Moreover, no exclusion according to healthcare level was applied, regarded the interest in collecting information on the effects of COVID-19 not only in hospital settings but also in primary care, where information is still scarce.
As far as medical research is concerned, children and pregnant women are special populations. Due to inherent ethical issues, data on these groups of patients is often scarce and less robust. This accounts for the choice made of excluding studies that comprehend these populations, for this scoping review was performed in a precocious moment of the COVID-19 pandemic. To minimize confounding information, studies comprising patients with previous coagulation disorders were also excluded, as they would have an increased risk of new thromboembolic events that could not be undoubtedly attributed to SARS-CoV-2 infection.
Some limitations regarding small population samples, simple comparisons, and brief follow-up period, due to the relatively short duration of the COVID-19 pandemic, were found, which resulted in low levels of evidence for many of the studies and, consequently, in a limitation for this scoping review.
The present synthesis of the published information contributes to a better understanding of coagulation disorders in patients with SARS-CoV-2 infection and of their characteristics, both in hospital and ambulatory settings.
Still, various uncertainties persist at all levels of the coagulation disorders approach in patients with SARS-CoV-2 infection. Thus, future research aimed to study the need for diagnostics, prophylactic, and treatment of coagulation disorders could promote and anticipate adequate care for those patients.
The existing information has its origin mainly in less robust sources, namely retrospective cohort studies, case reports or expert opinion, and in the hospital setting. This limitation is probably related to the novelty of the topic and the fact that serious cases and complications are referred to secondary care for observation and monitoring. However, considering the holistic approach and longitudinal follow-up of these patients by general and family medicine practitioners, it is important to encourage primary care physicians to share their experiences and results in scientific circles. Also, studies addressing the ambulatory setting would be of the utmost importance.
Thus, well-designed, randomized controlled clinical trials are needed to sustain clinical decisions at all stages.
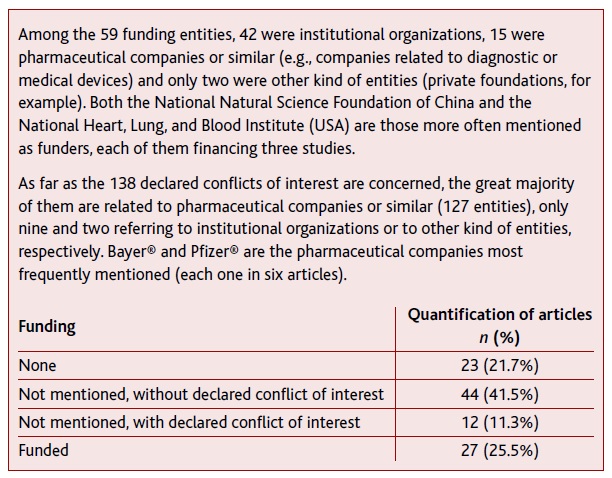
Funding
The present scoping review did not receive any specific funding. Table 5 summarizes information about funding and eventual conflict of interest mentioned in the included articles.