Introduction
Peripheral arterial disease (PAD) has become a major global health problem, affecting approximately 200 million individuals.1 Chronic limb-threatening ischemia (CLTI) is the most extreme manifestation of PAD and affects up to 11% of this population.2-4
The recent evolution in the vascular field gave rise to different solutions for the treatment of CLTI. Endovascular therapy has been experiencing a remarkable expansion with minimally invasive tools and techniques for arterial revascularization. The phenotypic expression of CLTI has changed in the last decades with the advent of diabetes mellitus as a global epidemic, resulting in an increase in the prevalence of tibioperoneal disease pattern.5-7 Distal disease is a therapeutic challenge to any surgeon, particularly for endovascular techniques.
Patients with CLTI in the setting of tibioperoneal disease are typically categorized as non-revascularizable when they lack an autologous vein graft and endovascular treatment had failed (or was considered technically unfeasible).8 Some centers, like ours, have accumulated experience in tibioperoneal revascularization using prosthetic grafts as an alternative to a lacking autologous vein conduit.8-10 However, to achieve acceptable results in distal prosthetic revascularization the presence of at least one excellent runoff vessel is mandatory.
This study aims to present the results of a surgical technique named inverted T bypass11 (firstly described as bridge graft technique by Deutsch el al12) which can be applied to patients with complex anatomic patterns and without an adequate autologous vein graft. This technique is useful for direct foot revascularization in situations that meet the following criteria:
- major ischemic foot wounds,
- an infrapopliteal artery with poor collateralization to the foot,
- an artery crossing the ankle/inframalleolar artery with poor run-off,
- a short venous graft with at least 3mm in diameter.
Briefly, an inverted T bypass consists in creating a bridge between both outflow arteries using a short venous graft that receives inflow from a heparinized prosthetic graft (figure 1). This bypass configuration distributes blood flow between the proximal and distal outflow arteries lowering run-off resistance, increasing prosthetic graft velocity and revascularizing two territories.
Methods
Study population
All patients with CLTI submitted to inverted T bypass between February 2017 and December 2022 were included. A retrospective analysis was performed in a single center with a limb preservation program. Baseline demographic and clinical characteristics, perioperative details and follow-up data were collected from medical records. All patients provided informed consent before surgery. The Institutional Review Board approval was waived for this study.
Classification systems
Wound and infection grade from the Society for Vascular Surgery WIfI staging system was determined to classify all wounds.13 Segmental femoropopliteal (FP) and infrapopliteal (IP) grades were determined from preoperative angiograms and reviewed by one of the authors. GLASS stage was determined combining FP and IP grades according to the literature.2
Preoperative approach
All patients were assessed according to the departmental protocol for CLTI. The vascular team performed a clinical evaluation including a dedicated duplex ultrasound to assess anatomic pattern, to preview the most plausible inflow and outflow vessels, and to evaluate the presence of an adequate venous graft. Preoperative angiography was performed to evaluate the extension of the disease (stratified by GLASS classification), to determine the most appropriate inflow and outflow vessels and to estimate the quality of the runoff. The patients that had no other possible solution, endovascular or conventional distal revascularization, and met the above-mentioned criteria were proposed for inverted T bypass.
Technique
Duplex ultrasound was performed on the day of the surgery to map and mark the vein segment and the outflow vessels. The vein was harvested first, to ensure the presence of an adequate graft. Inflow and outflow vessels were carefully dissected, preserving relevant collaterals. Anatomical tunneling was performed. Intravenous non-fractionated heparin was administered. Both distal anastomoses were performed without clamps using an Esmarch bandage. The first anastomosis was performed between the most distal outflow artery and the inverted venous graft. The second anastomosis was performed between the proximal outflow artery (most often the peroneal artery) and the opposite end of the venous graft. Afterwards, a termino-lateral prosthetic-venous anastomosis was performed close to the distal end of the vein graft (i.e., closer to the second anastomosis) to avoid the action of venous valves (figure 2). In our center the prosthetic graft used was a 6-mm heparin-bonded expanded polytetrafluoroethylene graft (Propaten®, W.L. Gore & Associates, Flagstaff, AZ). After completion of the prosthetic-venous anastomosis, the Esmarch bandage was removed. The final anastomosis was performed between the prosthetic graft and the inflow vessel (usually the femoral artery). All bypasses were controlled with intraoperative angiography.
Postoperative care
All patients initiated a protocol of a full dose anticoagulant therapy (warfarin with a target of INR 2.5 - 3.5 or, in non-compliant patients, direct oral anticoagulant) and lipid-lowering medication, the latter preferably initiated at least 1 week before surgery. Graft surveillance was performed at 1, 3, and 6 months after the procedure and every 6 months thereafter, consisting in physical examination and duplex ultrasound. Patients who failed to heal or with de novo signs of CLTI were promptly evaluated by duplex ultrasound and, if required, submitted to angiography and reintervention.
Clinical end points and variable definitions
The end points of the present study were limb-based patency (LBP), primary patency (PP) and secondary patency (SP) rates, freedom from CLTI, recurrence of CLTI, freedom from major limb amputation (or limb salvage), amputation free-survival (AFS), and overall survival. LBP was considered lost if anatomic failure (occlusion, critical stenosis, or any reintervention on the target artery path), or hemodynamic failure (clinical symptoms of CLTI in the index limb with significant stenosis on the target artery path). AFS was defined as patients alive without above ankle amputation of the index limb. Freedom from CLTI was achieved if AFS with complete wound healing and no ischemic rest pain was obtained within a period of 12 months. Recurrence of CLTI included all patients that achieved freedom from CLTI but developed a new wound or ischemic rest pain during the follow-up (as such, patients submitted to major amputation of the index limb during this period were considered a recurrence of CLTI).14 Chronic kidney disease was defined as an estimated glomerular filtration rate of less than 60 mL/min/1.73m2 determined with the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI).15
Statistical analysis
Statistical analysis was carried out using Stata 12.1 (StataCorp®, Lakeway Drive, College Station, Texas, USA). Continuous variables were presented as median with interquartile range from 25th to 75th percentile (IQR), and categorical variables were presented as absolute numbers with percentage values. Time-to-event end points were presented with Kaplan-Meier estimates with patients censored at major amputation, death, or last follow-up. All analyses were considered statistically significant if a two-tailed p value < .05 was observed.
RESULTS
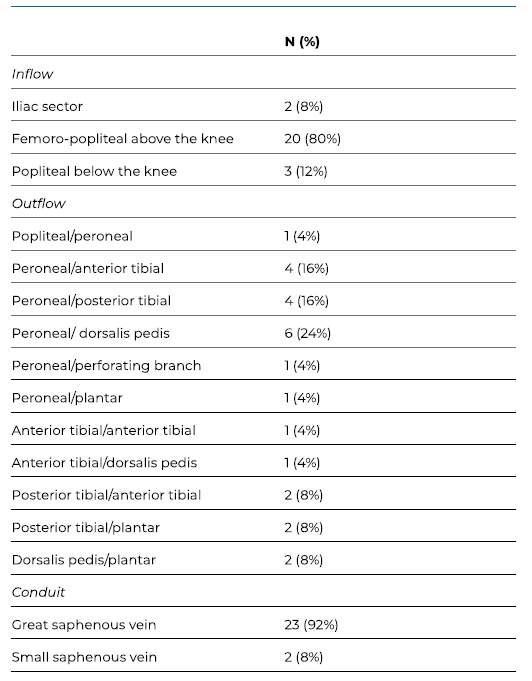
A total of 25 patients with CLTI were submitted to 25 inverted T bypasses. The majority of the patients were male (68%), with a median age of 77 years (IQR 17). Patients’ characteristics and clinical data are summarized in Table I. Twenty-four patients (96%) had hypertension, 72% had diabetes, 20% had history of smoking and 20% presented with end-stage kidney disease requiring dialysis. Four patients with an estimated glomerular filtration rate of less than 30 mL/min/1.73m2 (and not under dialysis) were not evaluated by preoperative angiography. Severe femoropopliteal and infrapopliteal anatomic patterns (GLASS FP and IP grade 4) were predominant, with rates of 57% (n=12) and 86% (n=18), respectively (see Table II). All limbs evaluated from preoperative angiographies (n=21) had GLASS stage III. Three patients (12%) had previously failed endovascular treatment.
Table I Clinical characteristics of patients submitted to revascularization using inverted T bypass operations.
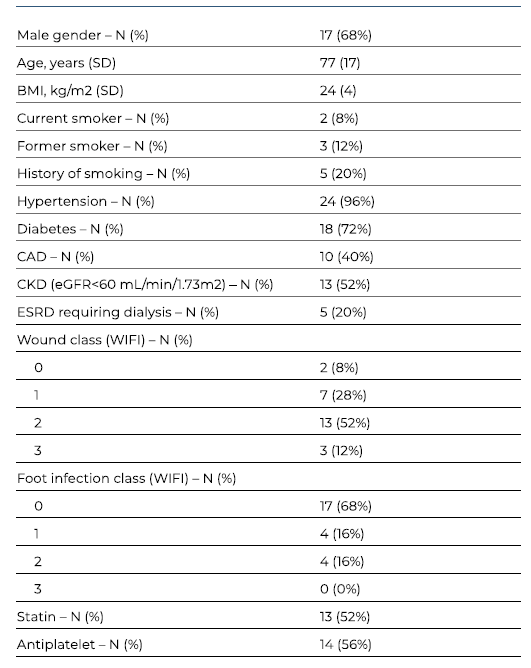
SD Standard Deviation; BMI Body mass index; CAD coronary artery disease; CKD Chronic Kidney Disease; ESRD End-Stage Renal Disease; WIFI Wound, Ischemia, and foot Infection Classification
Table II GLASS distribution determined from the 21 preoperative angiographies of patients submitted to revascularization using inverted T bypass operations.
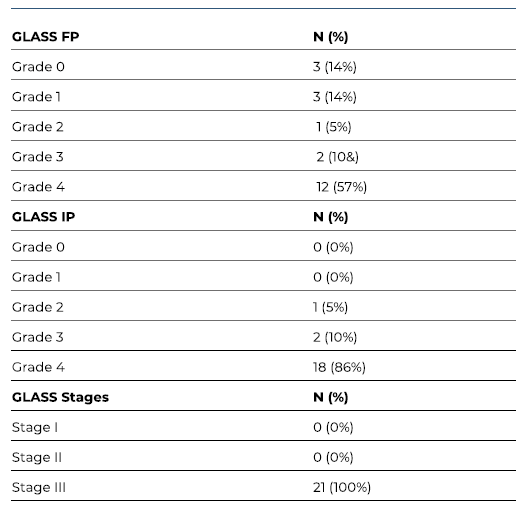
FP Femoro-popliteal; IP Infra-popliteal.
The peroneal artery was the most common proximal outflow vessel, targeted in 64% of the cases (n=16). Twenty-two (88%) inverted T bypasses obtained inflow from above the knee. Operative details are summarized in Table III.
The median follow-up was 25 months (IQR 32). LBP and primary patency had identical rates at 1 and 2 years (75% and 61%, respectively). Secondary patency rates were 79% and 64% at 1 and 2 years, respectively. Patency rates are summarized in Table IV and presented by Kaplan-Meier curves in Figures 3A-C. During follow-up, one early occlusion (< 30 days) was observed, from a total of nine. Four patients were reintervened, with one early occlusion. The remaining 3 reinterventions occluded at 5, 8 and 13 months.
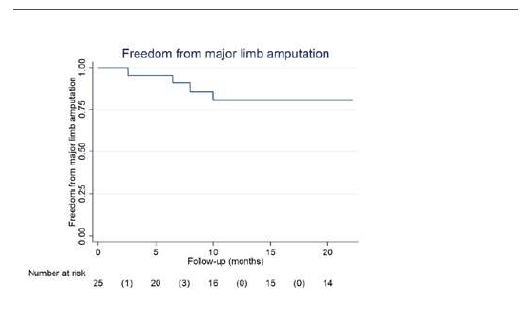
At 1 year, 86% of the limbs were free from CLTI (Figure 4A), and 79% of them remained without recurrences at 2 years (Figure 4B). During follow-up, 8 (32%) patients were submitted to major amputation, granting a 2-year freedom from major amputation of 81% (Figure 5). The overall survival was 71% at 2 years. All limb-specific and survival-related end points are summarized in Table IV.
Table IV Bypass-related and clinical outcomes
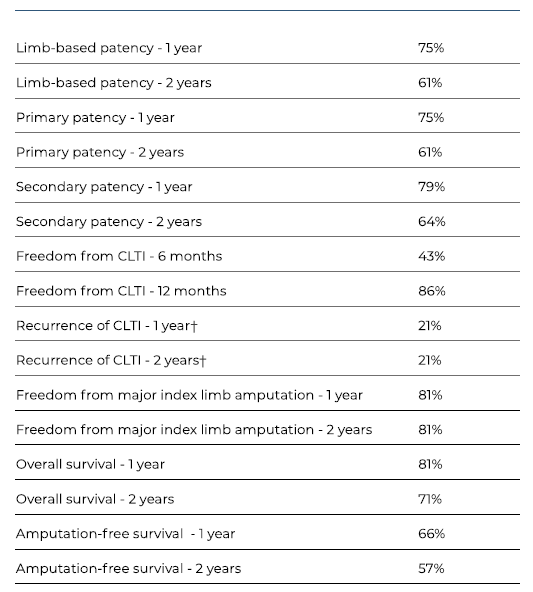
CLTI Chronic limb threatening ischaemia. ? Total of 17 cases, that is the total number of cases that was previously free from CLTI.
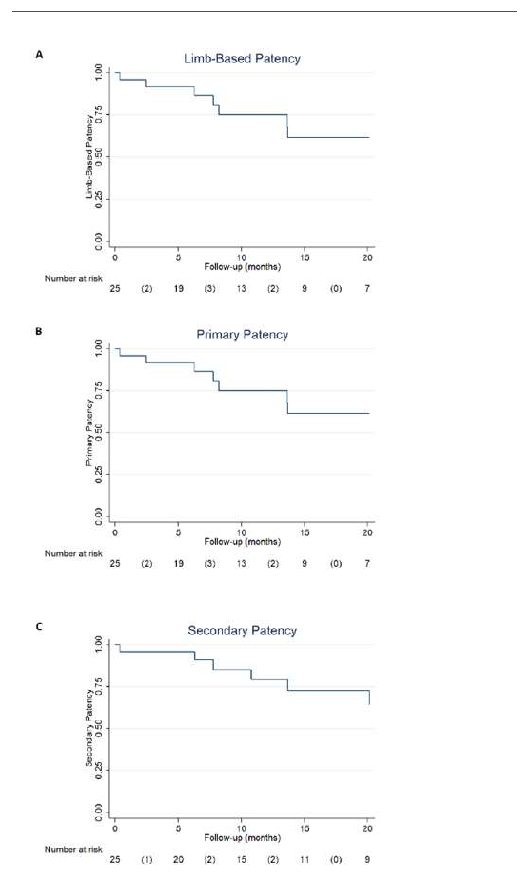
Figure 3 Kaplan-Meier curves depicting patency rates of inverted T bypass. A - Limb-based patency (LBP); B - Primary patency; C - Secondary patency.
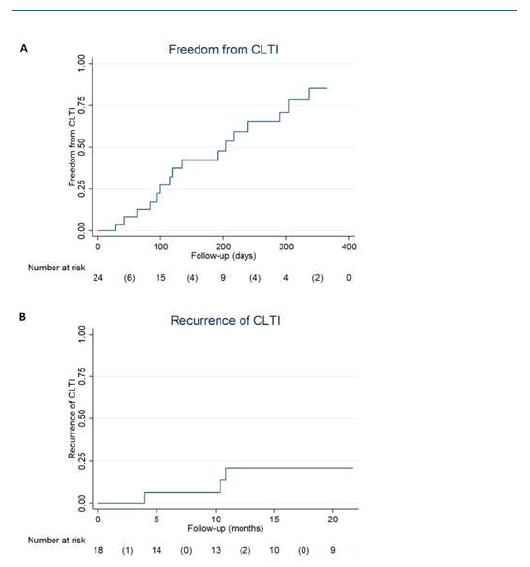
Figure 4 Kaplan-Meier curves depicting limb-specific end points of inverted T bypass. A - Freedom from CLTI; B - Recurrence CLTI.
Discussion
Open revascularization remains an important tool in modern vascular surgery. Global Vascular Guidelines recommend bypass surgery in patients with severe anatomic and clinical presentations (GLASS stage III and WIfI stage 3 or 4) when an adequate autologous vein is available.2 In these setting, endovascular intervention is considered the best alternative when venous graft is unavailable.2
The two most important limitations for bypass patency are inadequate autologous vein and poor outflow.2 Nevertheless, the absence of a good-quality venous graft is not an absolute contraindication for open surgery. Indeed, the results of the BEST-CLI trial, suggested that bypasses without a great saphenous vein graft appeared to achieve comparable outcomes to those of an endovascular intervention.4 Accordingly, several centers, including ours, have been accumulating extensive experience with prosthetic conduits, as an alternative to autologous vein, especially in the presence of good outflow.8,10,16
Inverted T bypass was performed for the first time in a complex multilevel occlusive disease case without a suitable vein and with two poor outflow vessels. To optimize the outflow the authors (DCS and GC) envisioned a short venous bypass between both outflow arteries. The premise is that two run-off vessels decrease peripheral arterial resistance and increase the ingraft flow velocity which, theoretically, optimizes the patency of the revascularization.11
Our results proved to be very promising considering the extreme cases presented: severe anatomic disease (100% GLASS III stage), absence of a full-length autologous vein and poor outflow vessels. Global Vascular Guidelines estimated a 1-year LBP <50% in patients categorized with GLASS III stage.2 We obtained LBP, primary and secondary patency rates of 61%, 61% and 74% at 2 years, respectively, which highlight the value of this technique. Additionally, most limbs were free from CLTI at 1 year (86%) and free from recurrence at 2 years (79%), granting a 2-year freedom from major amputation of 81%. These results seem to be comparable with a recent systematic review and meta-analysis of distal venous bypass that revealed 2-year PP, SP, and limb salvage of 78%, 87% and 83%, respectively.17 Another meta-analysis, focused on popliteal-to-distal vein bypass grafts, revealed 2 year-estimates of PP, SP, and foot preservation of 77%, 82% and 85%, respectively.18 As previously discussed, guidelines suggest endovascular approach in the absence of an adequate autologous vein graft. However, the current state of evidence is particularly limited regarding infrapopliteal angioplasty for the treatment of CLTI. A meta-analysis of distal angioplasty for CLTI showed 2-year PP, SP, and limb salvage rates of 51%, 64% and 84%, respectively.19 However, these results are lacking from studies with long-segment lesions. The few publications about limbs with long lesions (classified as TASC D) and submitted to infrapopliteal angioplasty presented modest results with 1-year PP rates of 37 - 48%.20,21
Inverted T bypass seems to be at least comparable to these different techniques of infrapopliteal revascularization. However, we must emphasize the anatomic complexity of our sample: (1) extensive occlusive disease with 100% of the cases categorized as GLASS III stage, (2) poor outflow vessels, and (3) no viable/failed endovascular solution. The inverted T bypass technique reserves the best conduit available for the most challenging territory - the tibioperoneal arteries.
The technical difficulties when performing an inverted T bypass are the same as in any distal bypass in patients with complex anatomic patterns and extensive calcified lesions. However, procedure duration is longer because it involves performing four anastomoses. This fact emphasizes the need for careful dissection and minimal blood loss, to reduce peri-procedure complications in these frail patients.
In conclusion, inverted T bypass is a creative surgical solution for the treatment of CLTI in patients with complex multilevel infrainguinal occlusive disease. The results are promising, rendering this technique a viable option for distal and ultra-distal revascularization in complex cases.