Introduction
Renal artery stenosis is a relevant manifestation of extra-coronary artery disease, with a prevalence from 1 to 5% in recent epidemiological series.1,2 Over 90% of all lesions are atherosclerotic and ostial. Renal artery stenosis leads to an overall renal hypoperfusion, renovascular hypertension and ischemic nephropathy.3) Renovascular hypertension occurs through continuous activation of the renin-angiotensin-aldosterone system induced by lower mean glomerular pressures and occur in unilateral or bilateral lesions.4,5) Ischemic nephropathy is a consequence of hypoperfusion-induced glomerular and interstitial lesion, and occurs in bilateral lesions or in a solitary kidney.6 Clinically, renal artery stenosis can range from asymptomatic forms to chronic renal failure needing renal replacement techniques, uncontrollable hypertension or flash pulmonary edema.7,8)
Apart from medical therapy, renal angioplasty was proposed as an effective treatment for patients with renovascular hypertension and acute renal failure.2,7,8) Several studies derived from institutional experience have been published and report that the technique is safe and effective. The experience from our institution was reported in 2019 and a significant effect on blood pressure control and renal function was observed.9)
Due to the limited level of this scientific evidence, two landmark clinical trials (ASTRAL and CORAL) were organized, and the results published in 2009 and 2014, respectively. Neither of these trials showed a clear clinical benefit by performing this technique.8,10) When compared with best medical treatment, patients who underwent endovascular procedures did not present a significant reduction in cardiovascular events nor did they have better blood pressure control or renal function improvement. These trials led to a paradigm shift in renovascular disease. However, they were criticized for methodological flaws, namely the inclusion of patients with less severe lesions, in a non-consecutive fashion, a high rate of complications as well as a selection bias introduced by the physicians’ criteria of selecting which patients to include.11,12
The aim of the present study is to evaluate the impact of these trials in our clinical practice and assess if the patients submitted to renal angioplasty before and after the CORAL clinical trial are clinically different.
Methods
This study followed the reporting guidelines from the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) Statement for cross-sectional studies.13
Study design:
We designed a cross-sectional study from patients submitted to renal artery angioplasty between 1999 and 2021 in a tertiary center. Patients were selected from electronical surgical records using the following ICD-10 diagnostic codes: “renovascular hypertension” (I15.0), “atherosclerosis of renal artery” (I70.1) or “hypertensive chronic kidney disease” (I12). The procedures and missing patients were confirmed and complemented with handwritten surgical records. Renal arteriograms with no further intervention, open renal artery interventions and renal graft failures were excluded from the analysis. Patients were divided in two cohorts: a historical control group already described in a previous study performed in this center9 (from 1999 to 2013, i.e pre-trial period) and a cohort of patients subjected to angioplasty from 2014 to 2021, i.e. post-trial period.
Patients were included in this series if they presented a greater than 60% stenosis in at least one renal artery, hemodynamically assessed by renal Doppler ultrasound. This lesion was later characterized either by computed tomography angiography or intraoperative arteriography. All patients underwent intervention in the setting of renal insufficiency, defined by glomerular filtration rate [GFR] <60 ml/min/1.73 m2) according to the updated 2021 CKD-EPI equations14 and/or difficult-to-control hypertension, i.e. arterial hypertension treated with two or more antihypertensive drug classes, according to the 2018 ESC/ESH Guidelines for the management of arterial hypertension.15 Hemodynamically significant lesions were assessed through the quantification of the maximum peak systolic velocity (PSV) and renal-aortic ratio (RAR). Stenotic lesions >60% were considered for a PSV>200 cm/s and RAR<3.5, or >80% if PSV>200 cm/s and RAR>3.5.
Procedure:
All patients were treated by percutaneous femoral access, and a "no-touch" technique was used to navigate and catheterize the renal arteries. A guiding catheter was used in most cases to stabilize the renal access and the lesions were crossed with 0.018 or 0.014 wires. Direct stenting was performed using balloon-expandable stents and no embolic devices were used. A control angiogram was always obtained before and after completing the procedure.
Data collection and follow-up:
All patients were assessed regarding demographic data, cardiovascular comorbidities (i.e. the presence of arterial hypertension, dyslipidemia, type 2 diabetes, coronary artery disease and cerebrovascular disease) and antihypertensive medication. Treated lesions were characterized according to their stenosis grade, (evaluated either through CT angiogram or Doppler ultrasound), location and bilaterality. Patients were followed for a minimum period of 12 months, starting from the day of the renal angioplasty. Patients were evaluated in clinical visits by the assistant vascular surgeon and nephrologist or assistant clinician (when appropriate) at 1 week, 1 month, 6 months and yearly. Serial blood samples were collected to assess renal function during follow-up through creatinine levels.
Study outcomes:
The primary outcomes of this study were the comparison between the 1999-2013 and the 2014-2021 cohorts regarding the median number of procedures per year and baseline comorbidities. Both cohorts were assessed regarding preoperative lesion severity, i.e. stenosis grade, bilaterality, lesion location. Preoperative glomerular filtration rate and blood pressure control, through the number of antihypertensive drugs, were compared between both cohorts. Both variables were assessed after renal artery angioplasty.
Statistical analysis:
The study cohort was divided in two groups, as previously mentioned. We performed a descriptive analysis of our data. Continuous variables were presented as mean (standard deviation) if normally distributed and median (interquartile range) if not. Dichotomous and categorical variables were expressed in numbers (percentage). Two-sample t-test or Mann-Whitney test was used when comparing continuous variables and Chi-Square/Fisher’s exact test to compare dichotomous variables. A paired t-test was used to compare the variation in glomerular filtration rate. Median follow-up time was assessed for both groups. All analyses were considered statistically significant if a two-tailed p-value < 0.05 was observed. Statistical analysis was carried out using STATA 16 (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC).
All patients provided informed consent before treatment.
Results
Impact of the trials in clinical practice:
After excluding patients subjected to open revascularization, diagnostic angiograms with no proven hemodynamically significant stenosis and patients with renal graft failure, a total of 152 patients were included.
104 patients were subjected to renal angioplasty between 1999 to 2013 (median number of procedures per year: 7, IQR 5-8), while 48 patients were subjected to endovascular therapy between 2014 and 2021 (median number of procedures 6.5 per year, IQR 4.5-7). When comparing both study periods, there was no statistically significant difference regarding the median number of procedures (p 0.53).
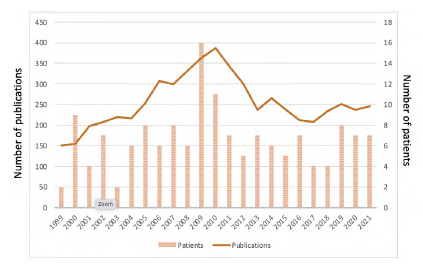
The ASTRAL and CORAL trials seemed to have a non-significant impact on our practice, when assessing the median number of procedures per year. However, a peak of interventions was observed between 2009 and 2010 (16 procedures in 2009 and 11 procedures in 2010). This surge in renal artery angioplasties is coincident with the publication of the ASTRAL trial results. After 2010, we identified a decrease to a baseline level of activity (Figure 1). Nevertheless, when performing a Poisson regression, the yearly number of renal artery interventions did not appear to be correlated with the study period (p 0.41).
Patient characteristics:
Baseline characteristics of both cohorts are reported in table 1. Overall, patients subjected to renal stenting are predominantly male (74.1%) and in their seventh decade. Most cardiovascular factors and comorbidities were homogenously distributed, apart from dyslipidemia, more prevalent in patients between 2014 and 2021 (47.9% vs 37.3%; p=0.005).
Chronic renal disease KDIGO 3-5 was more prevalent in the 2014-2021 group (83.3% vs. 45.2%; p < 0.001) and regarding blood pressure control, patients subjected to angioplasty between 2014 and 2021 were taking significantly more antihypertensive drugs in the preoperative setting (3 IQR 2-4 vs. 2 IQR 1-3; p 0.001). Patients subjected to stenting between 2014 and 2021 had a median preoperative worse renal function (eGFR 44,2 ± 25,9 vs. 68,6 ± 29,2; p < 0.001).
Renal lesion and severity and characteristics:
Renal artery lesion severity and baseline characteristics of both cohorts are reported in table 2. Over 90% of all lesions are ostial or proximal (90.8%), with no significant difference between cohorts (89.4% vs 93.8%; p 0.39). Mean stenosis grade was not statistically significant different in both groups (83.9 ± 7.14 vs. 84.6 ± 8.11; p 0.72).
When comparing the patients from our study with the ones enrolled in the CORAL trial,8) a significantly higher degree of stenosis was observed in our population (84.2 ± 7.52 vs. 72.5 ± 14.6; p < 0.001) as well as a significantly higher proportion of patients in stage 3-5 chronic kidney disease (56.6% vs. 49.6%; p 0.002).
Table 1 Baseline evaluation of the study cohort
| Total study cohort (n=152) | 1999-2013 (n=104) | 2014-2021 (n=48) | p | |
| Age (median (IQR)) | 69 (59-76) | 67 (56-74) | 70 (60.5-77) | 0.17 |
| Female sex | 38 (25.9%) | 26 (24.8%) | 12 (28.6%) | 0.69 |
| Comorbidities | ||||
| Hypertension | 142 (96.6%) | 100 (95.2%) | 42 (100%) | 0.31 |
| Dyslipidemia | 58 (38.2%) | 35 (33.3%) | 23 (47.9%) | 0.005 |
| Smoking history | 45 (31.6%) | 29 (27.6%) | 16 (33.3%) | 0.49 |
| Coronary artery disease | 36 (23.7%) | 28 (26.9%) | 8 (16.7%) | 0.16 |
| Diabetes | 32 (21%) | 20 (19%) | 12 (25%) | 0.42 |
| Chronic kidney disease KDIGO 3-5 | 86 (56.6%) | 47 (45.2%) | 40 (83.3%) | < 0.001 |
Numbers are presented as number of patients (proportion).
Table 2 Renal artery lesion characteristics in the study cohort
| Total study cohort (n=152) | 1999-2013 (n=104) | 2014-2021 (n=48) | p | |
| Bilateral renal artery stenosis | 34 (22.4%) | 24 (23.1%) | 10 (20.8%) | 0.76 |
| Solitary kidney | 7 (4.6%) | 4 (3.8%) | 3 (6.25%) | 0.39 |
| Lesion location | ||||
| Ostial/proximal third | 138 (90.8%) | 93 (89.4%) | 45 (93.8%) | 0.39 |
| Mid third | 11 (7.2%) | 9 (8.7%) | 2 (4.2%) | 0.26 |
| Distal third | 3 (2%) | 2 (1.8%) | 1 (2%) | 0.68 |
| Lesion severity | ||||
| Stenosis grade (mean±SD) | 84.2 ± 7.52 | 83.9 ± 7.14 | 84.6 ± 8.11 | 0.72 |
| Preoperative creatinine (mean ± SD) | 1.46 ± 0.81 | 1.27 ± 0.68 | 1.88 ± 0.93 | < 0.001 |
| Preoperative GFR (mean ± SD) | 60,9 ± 30,3 | 68,6 ± 29,2 | 44,2 ± 25,9 | < 0.001 |
| Preoperative number of antihypertensive drugs (median IQR) | 2 (1-3) | 2 (1-3) | 3 (2-4) | 0.001 |
Clinical success:
Despite significant differences between cohorts regarding preoperative renal function, we reported a significant improvement of GFR in patients subjected to renal artery angioplasty (70.4±3.4 ml/min vs 75.2±3.4 ml/min in the 1999-2013 group, p=0.02; 44.6±4.9 ml/min vs 49.6±4.6 ml/min in the 2014-2021 group, p=0.02). However, regarding blood pressure control, only 54% of patients reduced the number of antihypertensive drugs after renal artery angioplasty in the 1999-2013 cohort, while 47% of patients in the 2014-2021 cohort were able to reduce the number of drugs. No difference was found between cohorts (p=0.5).
Discussion
In this study, we concluded that after the CORAL trial our institution is treating more patients with more severe chronic renal failure and with more prevalent severe hypertension. This represents a significant change in practice and clinical indications for angioplasty. The study from Gupta and colleagues10 showed similar conclusions regarding the higher prevalence of associated manifestations of renovascular disease in the recent years with a significantly higher degree of stenosis than in the early years of the experience. This paradigm shift may reflect a more careful hemodynamic assessment of renal artery lesions.7 Furthermore, there was an increase in referrals from other specialties, particularly from the nephrology department. Patients were increasingly referred because of difficult-to-control secondary hypertension or acute-on-chronic renal failure.
Overall, there was not a significant reduction in the number of procedures per year, despite an increasing trend until 2009 followed by a decrease in activity to baseline levels. Indeed, a peak of interventions was observed between 2009 and 2010 (16 procedures in 2009 and 11 procedures in 2010). This surge in renal artery angioplasties is coincident with the publication of the ASTRAL trial results. Nevertheless, when performing a Poisson regression, the yearly number of renal artery interventions did not appear to be correlated with the study period (p 0.41). This trend was also identified in a consortium reported by Thomas and colleagues.16) Similar conclusions were drawn by Liang and colleagues from an analysis of data from a North American registry. 17 While the yearly number of procedures remained constant, there was a significant decline in the proportion of renal percutaneous interventions compared with interventions in other arterial beds. Among others, the authors relate this decrease to the results of the CORAL trial, as well as an improvement in best medical treatment.16,18) With the introduction of more effective antihypertensive therapies such as the renin-angiotensin-aldosterone blockade agents, most uncomplicated cases were controlled without the need for any intervention.2) Some studies report a reduction in mortality rates when under best medical treatment.19
Landmark clinical trials such as ASTRAL and CORAL refrained the interest in renal artery stenting and consequently the number of interventions, with disappointing clinical outcomes compared with best medical treatment. However, when comparing these results with your experience, patients enrolled in our study cohort had significantly higher renal artery stenoses (84.2 ± 7.52 vs. 72.5 ± 14.6; p < 0.001) and a significantly higher proportion of patients in stage ≥3 chronic kidney disease (56.6% vs. 49.6%; p=0.002). This inclusion bias may have contributed to the disparity in results. Indeed, further studies have focused in the inclusion of hemodynamically significant lesions in consecutively treated patients.20,21 In these studies, more careful patient selection contributed to a more significant benefit for these patients.
This study is one of the first showing the treatment trends on renal artery stenosis, with particular emphasis to the impact of the CORAL trial and how it was a turning point. Moreover, by including consecutive patients over the last two decades, it offers a real-world analysis. Finally, all lesions were objectively assessed as hemodynamically significant, with angiographic confirmation.
The study presents some limitations. Firstly, its retrospective design contributes to an information bias and data collection issues. Furthermore, blood pressure control was primarily assessed through the number of antihypertensive drugs. Accurate and serial measurements of blood pressure would be an interesting feature to introduce in further studies. Ideally, renal function should be evaluated by more reliable markers or assessed in renal scintigraphy. Further studies should be prospective and additionally address clinical outcomes, such as major adverse clinical events (MACE) and the impact of renal angioplasty.
Conclusion
Renal artery angioplasties emerged as a significant and less invasive tool in the treatment of renal artery stenosis in patients with renovascular hypertension or renal failure. Nevertheless, the publication of the CORAL trial in 2014, while showing no clear clinical benefit when comparing to best medical treatment, halted the clinicians’ enthusiasm on percutaneous interventions with an impact in clinical practice. Our cross-sectional study compared two real-world cohorts before and after the publication of this landmark trial. While there was no significant decline in the yearly number of procedures, patients treated after 2014 had more severe renal artery lesions, as well as worse preoperative renal function and blood pressure control. Further studies should assess prospectively clinical outcomes in this real-world setting to understand who benefits from this procedure and how we should interpret the results of these clinical trials.
Acknowledgements: None
Conception and design: AD, MM, AL, GS, LMP
Analysis and interpretation: AD, MM, LMP
Data collection: AD, MM
Writing the article: AD, MM, LMP
Critical revision of the article: AD, MM, AL, GS, LMP
Final approval of the article: AD, MM, AL, GS, LMP
Statistical analysis: AD, MM
Obtained funding: Not applicable
Overall responsibility: AD, LMP
Conflicts of interest: None
Funding: None