Introduction
AEF following TEVAR is a rare entity with an incidence of 1.5-1.9%.1-8 Patients usually present at an emergency unit with gastrointestinal (GI) haemorrhage (haematemesis) and constitutional symptoms such as fever, usually of unknown origin.1,2,8 If untreated, or treated conservatively, this condition carries a mortality rate close to 100%.8,9 Awareness of this condition, celerity of diagnosis and early surgical treatment increases the likelihood of survival in this population of patients.1,3,8,9
Case report
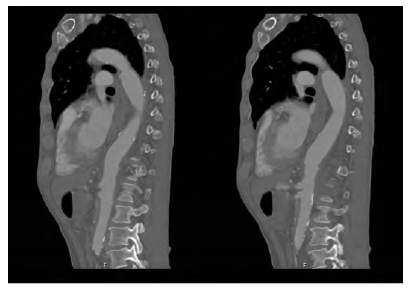
A 62-year-old man, heavy smoker, presented at the emergency department with dysphagia lasting for one month. Based on computed tomography (CT) he was diagnosed with a saccular descending aortic aneurysm and was electively submitted to TEVAR, promptly resolving his clinical complaints, and discharged. One-month after the endovascular procedure, again in an emergency setting, he returns to our centre complaining about chest pain and haematemesis. At presentation, the patient was hemodynamically stable. The CT scan showed ectopic gas inside the aneurysmatic sac (Figure 1) and dilation of the TEVAR landing zones leading to a deficient apposition of the stent graft proximally and distally. He then underwent an oesophagogastroscopy in which a segment of the stent graft was visualized. No hemorrhagic foci were identified in both exams.
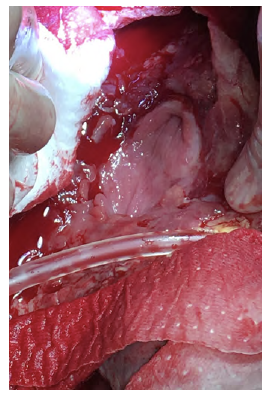
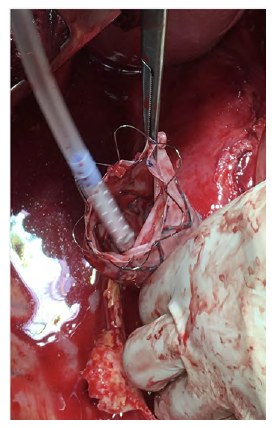
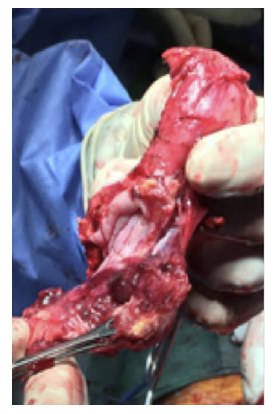
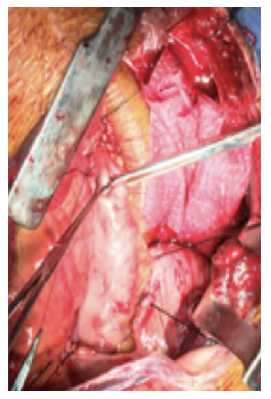
After a multidisciplinary discussion, a staged approach was decided. The first step aimed for oesophagus defunctionalization, by performing cervical oesophagus stapling, a drainage gastrostomy, and a feeding jejunostomy. After a two-week course of broad-spectrum antibiotics and antimycotics the patient underwent the definitive surgical treatment. A left lateral thoracotomy was performed, exposing the descending aorta and the oesophageal fistula (Figure 2). The stent graft was explanted (Figure 3), extensive debridement was followed by in situ reconstruction of the descending aorta with an aortic interposition of a silver and Triclosan impregnated Dacron graft (Figure 4), and lastly by esophagectomy (Figure 5), and eosophagogastrostomy (Figure 6). An intercostal muscle flap was used to fill the aortoesophageal space, to cover the graft and to take advantage of its neovascularization potential. The postoperative period elapsed without any major complications and the patient was discharged after 30 days. Antibiotic and antifungal drugs were administrated until discharge.
After four years of follow-up the patient remains asymptomatic. Follow-up CT showed no signs of reinfection or de novo fistulae and confirmed the patency of the aortic and oesophageal reconstruction (Figure 7). However, during this time, three endoscopic oesophageal dilations of the cervical eosophagogastric anastomosis were needed for isolated periods of dysphagia recurrence.
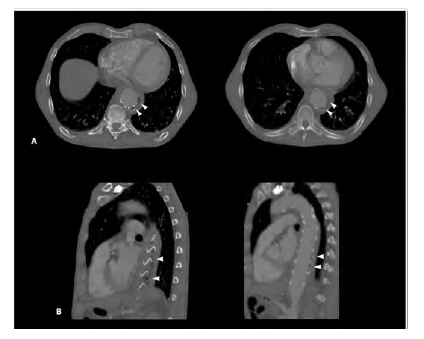
Figure 1 CT angiography of the descending thoracic aorta showing the TEVAR stent graft surrounded by air (arrowheads) in an axial (A) and sagittal (B) planes.
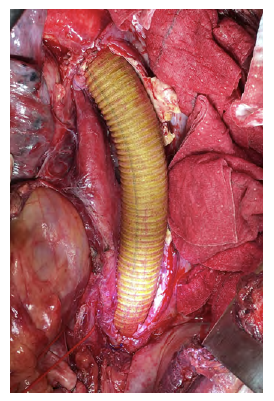
Figure 4 Descending thoracic aortic in situ interposition with silver and Triclosan impregnated Dacron graft.
Discussion
Although rare, AEF can have a devastating outcome1,4,5,8,9 Despite the vast differential diagnosis, any patient presenting with GI bleeding and with a history of previous descending aortic surgery should undergo an expeditious evaluation for AEF. In this population, this entity should always be excluded before any other cause of bleeding.1 According to recent studies, the median time until diagnosis of an AEF was 90 days.2,6,8
The mechanisms thought to lead to the fistulization process of a secondary AEF following TEVAR are still not completely clear. Most authors suggest the following as the underlying reasons:1,2,3,7 primary infection of the endoprosthesis, erosion of the aortic and oesophagic wall by the stent graft (possibly related to excessive oversizing), ischemic necrosis of the oesophageal wall by covering the oesophagus feeding arteries and endoleak into the residual aneurysm sac.
A high index of suspicion is paramount. The typical clinical findings are chest pain, sentinel bleed and exsanguination in extreme cases.1,2,3 Constitutional symptoms like fever, anorexia and tiredness are also common.1,2,8 A herald bleed is present in more than half of the patients and may represent the perfect timing for intervention, as exsanguination is impending.1
CT and endoscopic studies are the most useful image studies available1,3 CT is the mostly commonly used and establishes the diagnosis frequently by detecting air surrounding the stent graft, although it rarely detects the fistulous tract.2,8 Oesophagogastroscopy not only identifies the culprit lesion but also allows direct visualization of the stent graft is some cases. Sometimes a clot can hinder these findings and biopsies should not be attempted.1
Management remains controversial. Conservative management seldomly is effective, with 1-year mortality rates reaching 100% in the majority of cases.1,2,8 The primary concern, in an unstable patient with active gastrointestinal bleeding, is to prevent exsanguination. In this setting, TEVAR can be considered as a bridging procedure until the patient is stable enough to undergo the definitive surgical treatment, the latter being the only potential cure.3,4,6,8 During this time period, antibiotic and antimycotic drugs should be administered for optimal control of the infection.2,4,5,6,8
According to the published literature, an aggressive surgical treatment offers the best survival rates.8 To diminish the morbimortality related to the procedure, patients typically follow a staged approach. Initially the clinician should focus on controlling the bleeding and optimal control of the infection, including oesophagus defunctionalization, later being followed by the reconstruction of the aorta and the GI tract. Infection control is a concern and, alongside with antimicrobials, surgical debridement of the diseased oesophagus and aorta should be guaranteed. Primary repair of the oesophageal defect is a possibility in minor lesions without mediastinitis.2 The stomach or colon can be used when a GI tract reconstruction is needed. Vascular reconstruction of the aorta is also mandatory by explantation of the endoprosthesis and aortic reconstruction, in situ or by extra-anatomical bypass. Omentopexy or the use muscle flaps should be encouraged, covering the graft and the GI reconstruction, as these highly vascularized tissues promote angiogenesis, enhancing the immune response and bacterial clearance, hence preventing reinfection.2,4,8 Temporary feeding devices, via gastrostomy or jejunostomy, are a rule.2,3,5,7 These approaches are only possible with a capacitated multidisciplinary team and proper equipment, something that only a minority of centers can offer.
Our case report focuses on a patient that presented to our emergency unit quite early after TEVAR (<90 days), complaining of chest pain and GI bleeding. He inevitably underwent a CT and oesophagogastroscopy and the diagnosis of an AEF was expeditious. He was offered a staged, aggressive, and multidisciplinary medical and surgical approach. An in situ reconstruction of the descending aorta was decided based on the absence of local inflammatory and infectious signs, preceded by a two week course of antimicrobials. The preference for a prosthetic graft instead of a cryopreserved aortic homograft was due to unavailability of the latter. Despite the prosthetic reconstruction, the patient remains asymptomatic and clinically stable after four years of follow up.
Conclusion
AEF usually presents early after TEVAR and a high index of suspicion is paramount to achieve an expeditious diagnosis. A prompt multidisciplinary approach is imperative. Improved disease-free survival can be obtained despite the precarious preoperative conditions and complex anatomical features, commonly found in this group of patients.