Introduction
As vascular surgery progresses and becomes less invasive, there is a growing number of lower limb revascularizations performed in high-risk patients with heart disease.
Cardiac co-morbidities are common in patients with lower limb ischemia, since peripheral arterial disease (PAD) and coronary heart disease and some heart valve diseases share similar atherosclerotic risk factors.
It is known that PAD patients have a twofold increase in the prevalence of congestive heart failure (CHF) and are at high risk for major cardiovascular events(1-5).
There have been reports that CHF is associated with increased medical perioperative complications(1). However, the impact of heart disease on limb outcomes in patients that underwent lower limb revascularization is not well established.
Inadequate systemic perfusion as a consequence of heart disease may compromise inflow after lower limb revascularization procedures, decreasing their short and mid-term patency(1,2). Additionally, heart disease may be associated with platelets, endothelial and vascular smooth muscle cell dysfunction, leading to an increased propensity for vascular procedure thrombosis(1,6).
It may be theorized that patients suffering from significant heart valve disease or reduction of left ventricular ejection fraction (LVEF) have worse limb outcomes after lower limb revascularization.
This study aims to compare long-term limb and life outcomes in patients with moderate or severe heart disease to those without.
Methods
This is a retrospective, observational, single-center study. It includes all first lower limb revascularization procedures performed in a tertiary university hospital, between January 2017 and December 2018, in patients with diagnosed PAD and an available preoperative transthoracic echocardiogram (TTE). Patient, procedure and outcome details were collected from medical records. It included analysis of admission and discharge documents, hospitalization and outpatient registries, and review of available follow-up exams (ultrasound, CTA or invasive angiography). In the presence of chronic limb-threatening ischemia (CLTI), limbs and wounds were classified according to WIfI (wound, ischemia, foot infection) system, and PAD was staged according to GLASS (Global Limb Anatomical Staging) system after arteriography evaluation by the authors. TTEs were reviewed in order to determine the baseline LVEF and the presence and severity of heart valve disease. We considered any TTE performed in the 2 years period that preceded the vascular procedure.
Patients were categorized into two groups according to presence and severity of heart valve disease and LVEF.
We used the echocardiographic criteria shown on Table 1 to grade the heart valve disease and the LVEF.
Group 1 included procedures in patients with moderate to severe heart disease on TTE, defined as LVEF<40% or moderate to severe valvular heart disease.
Group 2 included procedures in patients with mild or no heart disease on TTE, defined as LVEF≥40% and mild or no valvular heart disease.
Group 1 was further stratified according to presence and severity of the individual heart change on TTE. In that way, we evaluated separately:
Moderate (LVEF=30-39%) and severe LVEF reduction (LVEF<30%);
Moderate and severe aortic valve stenosis and regurgitation;
Moderate and severe mitral valve stenosis and regurgitation;
Moderate and severe pulmonary valve stenosis and regurgitation;
Moderate and severe tricuspid valve stenosis and regurgitation.
The primary endpoint of this study was major amputation, and secondary endpoints were diagnosed vascular procedure restenosis/occlusion, vascular reintervention and overall survival.
Diagnosed vascular procedure restenosis/occlusion was determined on follow-up exams, including ultrasound, CTA or invasive arteriography. In authors' department, patients undergo vascular surgeon observation and lower limb vascular ultrasound on an outpatient basis a month after discharge, six months after discharge, yearly after that, and every time symptoms recur or become more severe. This protocol is followed after open, endovascular or hybrid revascularization procedures. If the patients have healing wounds, they are clinically followed closer, usually every two weeks.
A vascular reintervention was defined as a vascular revascularization procedure performed on the same limb. It included procedures performed: 1) after a diagnosis of vascular procedure restenosis/occlusion associated with life-limiting claudication or CLTI; 2) after a diagnosis of asymptomatic failing-graft; 3) after vascular procedures that did not relieve previous symptoms, despite having treated arterial anatomic lesions; 4) after symptoms recurrence due to atherosclerosis progression in another sector.
Quantitative variables are expressed as mean ± standard deviations (SD) or as median (interquartile range - IQR), as appropriate. Qualitative variables are expressed as absolute values and percentages. Shapiro-Wilk normative tests were used to access distribution pattern in quantitative variables. Student's t test, A-nova one way and respective non-parametric tests and χ2 and proper adjustments were used on univariate analysis. Correlations tests have been applied when relating quantitative variables. A p-value <0.05 was considered statistically significant. Statistical analysis was performed using SPSS 24.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
Between January 2017 and December 2018, our department performed 420 first lower limb revascularization procedures. This study included 268 vascular procedures with an available preoperative TTE (64%).
The specific change in TTE is shown in Table 1.
Table 1 TTE diagnosed heart disease and echocardiographic criteria
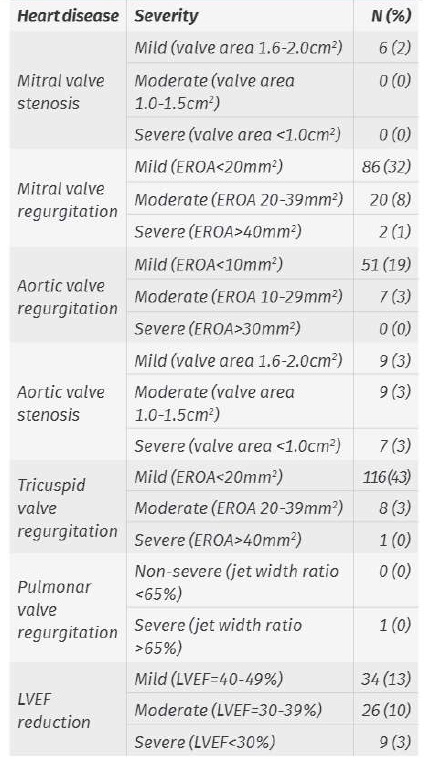
Note: some procedures were performed in patients with more than one TTE change
EROA: effective regurgitant orifice area
Group 1 included 70 procedures. 30% were performed in women (N=21) and 70% in men (N=49).
Group 2 included 198 procedures. 20% were performed in women (N=40) and 80% in men (N=158).
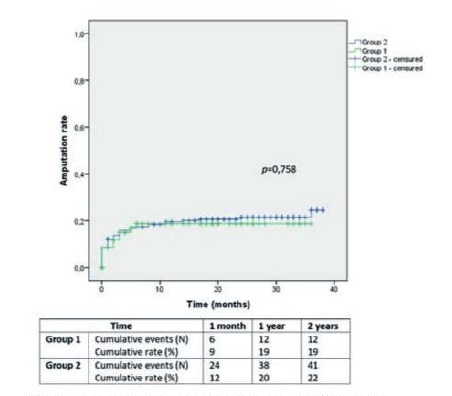
Group 1 included older patients than Group 2 (p=0,013). Group 1 and 2 procedures were performed in patients with a mean age of 71,3±9,3 and 67,7±10,3 years, respectively. Baseline characteristics are depicted in table 2. There were no other significant difference between groups, aside from a higher prevalence of dyslipidemia in Group 1 (63% vs. 43%; p=0,004) and of coronary disease or CHF in Group 1 (59% vs. 38%; p=0,003).
Group 1 included 35 procedures in patients with LVEF<40% and 46 procedures in patients with moderate to severe valvular heart disease.
In both groups, the indication for treatment was CLTI in 89% (N=62 and N=177 in Group 1 and 2, respectively). Group 1 and 2 procedures were performed due to lifestyle-limiting claudication in 11% (N=8 and N=21, respectively; p=0,849).
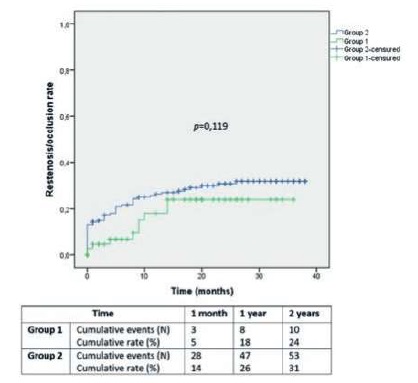
If CLTI, there were no significant differences between Groups 1 and 2 in wound and infection grading (in WIfI classification), and in anatomic disease staging (aortoiliac, common femoral artery, femoropopliteal, BTK and BTA disease in GLASS; Table 3). After stratifying Group 1 with CLTI, we found higher grades of infection (WIfI) if LVEF<40% (p=0,013).
We found no difference between Groups 1 and 2 in the type of revascularization procedure performed (Table 4; p=0,34). Group 1 included 73% endovascular procedures (vs. 65% in Group 2). The difference remained non-significant after stratification in subgroups.
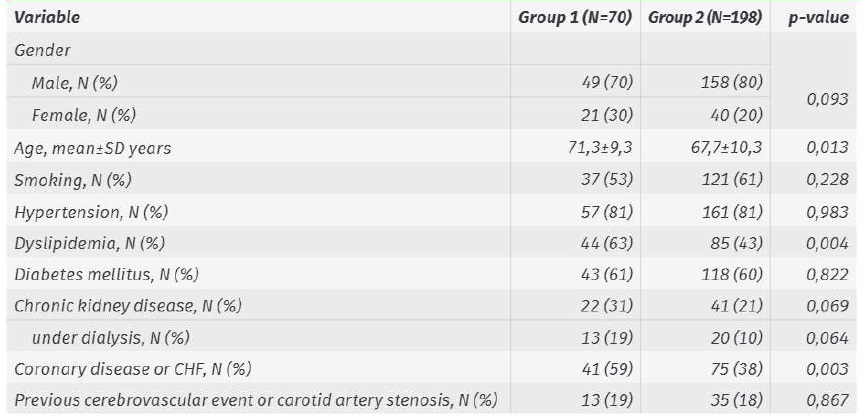
Major amputation rates in Group 1 and 2 were 9±3% and 12±2% at 1 month, 19±5% and 20±3% at 1 year and 19±5% and 22±3% at 2 years, respectively (Image 1; p=0,758).
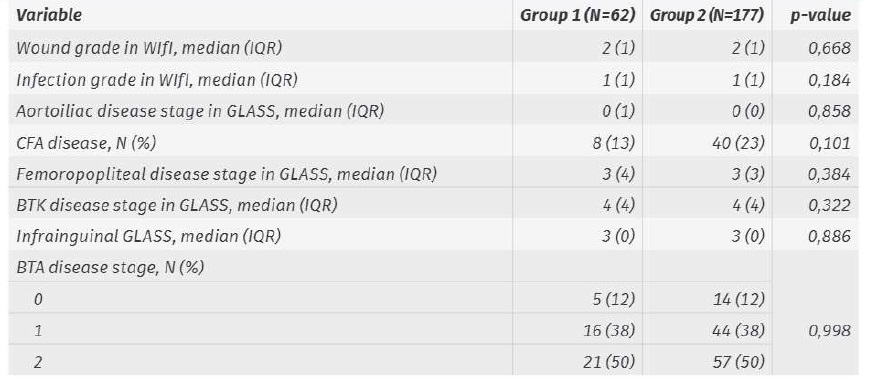
Diagnosed procedure restenosis/occlusion rates in Group 1 and 2 were 5±3% and 14±3% at 1 month, 18±6% and 26±3% at 1 year and 24±7% and 31±4% at 2 years, respectively (Image 2; p=0,119).
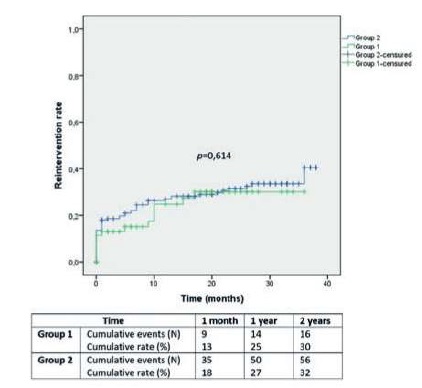
Vascular reintervention rates in Group 1 and 2 were 13±4% and 18±3% at 1 month, 25±6% and 27±3% at 1 year and 30±7% and 32±4% at 2 years, respectively (Image 3; p=0,614).
After subgroup analysis according to the presence and severity of individual heart change, the difference remained non-significant for the above-mentioned outcomes.
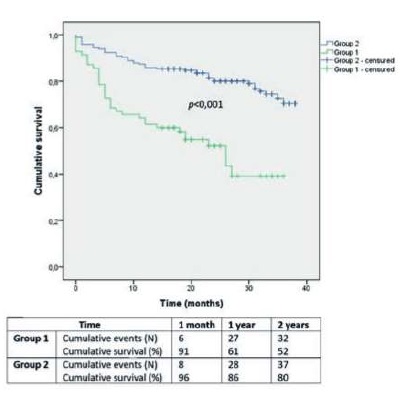
Overall survival in Group 1 and 2 was 91±3% and 96±1% at 1 month, 61±6% and 86±3% at 1 year and 52±6% and 80±3% at 2 years, respectively (Image 4; p<0,001).
After stratification, LVEF<40% was associated with a dismal overall survival (39±9% at 2 years), comparing to LVEF≥40% (78±3% at 2 years; p<0,001). Moderate to severe heart valve disease had also a worse survival prognosis (59±8% at 2 years), comparing to mild or no heart valve disease (76±3% at 2 years; p=0,004).
Discussion
How the presence of heart disease affects limb revascularization's outcomes has not been clearly demonstrated in literature.
Our study suggests that, despite worse survival, the outcome of lower-limb revascularization procedures in patients with moderate to severe left ventricular dysfunction or heart valve disease is similar to that of patients without heart disease. This is a relevant and unexpected finding.
Due to the presence of similar risk factors and underlying pathophysiology, patients with PAD commonly have pre-existing changes in TTE.
Most of known studies on limb outcomes after revascularization that consider heart failure as a possible modulator focus on clinical criteria(1,7,8). Only one study considered LVEF(6) and there are no studies considering heart valve diseases. Using TTE, we could classify heart disease by specific objective criteria.
As the prevalence of heart failure from all causes rise with aging population, it is expected that patients with heart disease in TTE would be older, as in this study(1). We found no significant difference in clinical presentation or anatomic pattern of PAD in patients with or without major heart disease in TTE.
Heart failure is a known risk factor for perioperative morbidity and mortality after major surgery. We could expect that patients with CHF and comorbidities frequently associated with it should preferentially be offered endovascular intervention instead of surgical bypass either for severe claudication or CLTI(6). However, in this study, the procedure choice was not associated with presence of preoperative TTE changes. Perioperative care of high-risk patients in intermediate or intensive care units can be an explanation.
A prior study showed that CHF is not associated with decreased patency for femoro-popliteal or femoro-tibial bypass grafts but was associated with postoperative medical complications and higher mortality(1). Another study found no association between coronary heart disease and heart rhythm disorders and prosthetic above-knee bypass patency(7).
However, other studies, showed that endovascular interventions in patients with CHF are associated with reduced patency(6,8). Meltzer et al. showed that CHF with EF<40% was an independent predictor of reduced primary patency and secondary patency, and it was associated with worse limb salvage(6).
Our study does not establish any clear relation between limb outcomes and the presence of TTE major changes. In fact, patients with moderate to severe heart valve disease or reduced LVEF had no different amputation rates, diagnosed restenosis/occlusion rates and reintervention rates than patients with minor or no changes on TTE.
It is known that the diagnosis of heart failure is associated with increased perioperative risks and poor survival(1-3). In fact, in the presence of CLTI, the associated diagnosis of heart failure increases nearly by twofold the mortality and major adverse cardiac or cerebrovascular events(2). In this study, any TTE major change, LVEF<40% and moderate to severe heart valve disease were all associated with poor mid to long-term survival.
This study suggests, as other ones, that cardiovascular risk reduction and heart disease management optimization in patients with lower limb ischemia is needed to improve outcomes in this high-risk population(1,2).
We identified some limitations in our study. It is based on medical registry and patient clinical and imagiological follow-up. The cardiac examinations were made by several operators in different laboratories so there may be operator discrepancies, but this is likely to equally affect both groups. Our study did not included patients with heart failure with a preserved LVEF. A selection bias may be present, as an unknown number of patients with heart failure or heart valve disease may have been deemed unfit for surgery due to higher operative risk. Our department protocols do not include systematic measurements of ankle-brachial index, ankle pressure or other criteria to evaluate ischemia grade in WIfI. Vascular procedure selection is dependent on surgeon experience. Its relatively small sample does not allow performing a multivariate analysis to eliminate the role of possible confounders affecting outcomes.
Conclusion
Our study suggests that moderate to severe heart disease, detected in TTE, does not influence limb-related revascularization outcomes. However, patients with heart valve disease or LVEF reduction have worse overall survival. We should not expect worse limb outcomes in patients with heart disease, but aggressive tertiary prevention should be provided to improve vital prognosis.