Introduction
Periodontitis is a multifactorial infection that results in connective tissue destruction, alveolar bone resorption, and, eventually, tooth loss.1 Periodontal disease is one of the most prevalent chronic inflammatory diseases worldwide. Non-surgical treatment has been effective in most cases, but it has some limitations.1 Recently, an adjuvant for periodontal treatment that could improve periodontal healing has been studied. A wide range of chemotherapeutic agents, chlorhexidine and, more recently, hyaluronic acid (HA) have stood out.2
HA is one of the main components of the matrix of the periodontal ligament. It assumes a multifunctional role in wound healing, has a great anti-inflammatory capacity, and plays a role in adhesion, reproduction and cell proliferation.3
Its topical application has recently been used as an adjuvant treatment for chronic inflammatory diseases.3 Different local antimicrobial and anti-inflammatory adjuncts have been shown to improve the outcome of scaling and root planning (SRP).
Chemical agents are widely used to treat oral diseases, and various antibiotics and anti-inflammatory agents have been trailed in previous studies.3 However, recent initiatives have started to use chemotherapeutic agents to treat periodontal diseases, and HA is a recent addition to those.3 HA has been used as an exogenous agent in the treatment of chronic inflammatory changes due to its role in the control of inflammation and tissue regeneration mechanisms. Since HA is non-toxic, biocompatible, and has numerous biochemical and physio-chemical features, its topical and systemic application benefits the host response regulation.4
HA has already been used in the treatment of inflammatory processes in various domains, such as orthopedics, dermatology, and ophthalmology. In dentistry, it has been used in temporomandibular joint disorders, and more recently in the treatment of periodontal disease thanks to its anti-inflammatory, anti-edematous and anti-bacterial effects.5
HA has two essential functions: play a structural role, due to being a constituent of the tissues’ architecture that increases the volume of the extracellular matrix (ECM) by filling it with water and ions; and regulate cell signalization, as it interacts with constituents of the ECM and with membrane receptors.6
HA can directly prevent the proliferation and colonization of periodontal pathogens and indirectly moderate inflammation and stabilize the granulation tissue by preventing degradation of the extracellular matrix proteins by serine proteinases derived from inflammatory cells as healing progresses.5
Indeed, preliminary clinical trials have shown that HA has anti-inflammatory, anti-edematous, and anti-bacterial effects for the treatment of gingivitis and periodontitis. It plays an anti-inflammatory role by inhibiting tissue destruction and facilitates healing by reducing prostaglandins, metalloproteinases, and other bioactive molecules.7
The purpose of this systematic review was to evaluate the clinical effects of local subgingival use of 0.8% or 0.2% HA when adjunct to SRP in patients with chronic periodontitis (CP).
Material and methods
This review was registered with the identification number CRD42019131541 in the PROSPERO International Prospective Register of Systematic Reviews hosted by the National Institute for Health Research, University of York, Centre for Reviews and Dissemination.
An electronic search without time or language restrictions was undertaken between January and May 9, 2018, in the following databases: PubMed, Lilacs, and Cochrane, using the following keywords and Mesh terms: “hyaluronic acid”; “periodontitis; “treatment of periodontitis”; “scaling.”
In this systematic review, only randomized controlled clinical trials (RCTs) on the treatment of CP with SRP + HA were included. Either the test or control treatment group of each selected RCT was included in the article if the following inclusion criteria were met: healthy participants; moderate to severe CP with ≥ five sites of probing pocket depth (PPD) ≥ 5 mm; patients with at least 20 teeth; follow-up ≥ 12 weeks; human-based studies; HA applied during the non-surgical treatment of CP. Individuals were excluded if they had received periodontal treatment in the previous year and/or antibiotics 6 months before the study, were smokers, had chronic diseases (diabetes mellitus or rheumatoid arthritis), or were pregnant.
This systematic review applied the Preferred Reporting Items Systematic review and Meta-Analyses (PRISMA) statement and checklist, as well as the population, intervention, comparison, outcomes (PICO) method, as follows:
• P: Patients with CP
• I: SRP + HA
• C: Treatment of CP with either SRP + HA or SRP alone
• O: Clinical attachment level (CAL) gain, PPD, bleeding on probing (BOP), plaque index (PI), gingival index (GI), and relative attachment level (RAL)
The risk of bias analysis was performed with the Cochrane Collaboration Tool, by two authors (VR and PO). The risk of bias was classified as low when studies provided detailed information on all parameters analyzed. Studies that did not provide information on two or more parameters were considered as high-risk. Studies that provided information on only one of the parameters were classified as having a moderate risk of bias.
All the included trials were described as randomized controlled trials, and all of the studies showed an unclear risk of the randomization method. Allocation in three of the included trials was classified as unclear since the method of allocation was not described.2,8,9In two of the studies included allocation was classified as high-risk10,11and in other two as low-risk.12,13
Blinding for participants was well conducted in three trials;8,12,13the other trials did not have all the information required for this analysis, so they are classified as unclear2,9-11 and considered as having an unclear risk for blinding bias at outcome measuring. All of the included studies reported follow-up and whether the proposed outcomes were assessed, as described in the protocol. Subsequently, most studies, after the risk of bias assessment, were classified as having a high risk of bias.
Results
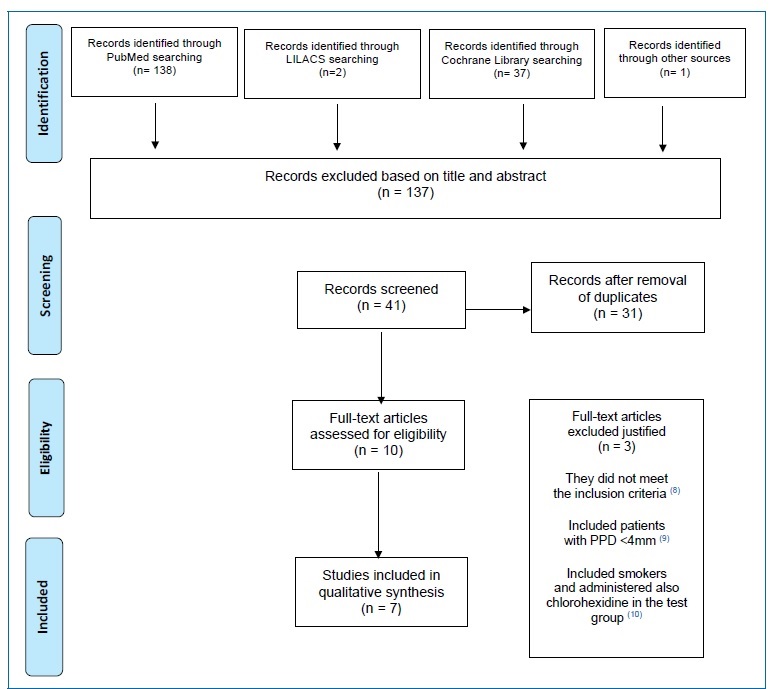
The study selection process is summarized in Figure 1. The search strategy resulted in seven papers. All data were extracted by two reviewers (VR and PO), independently and in duplicate, using data extraction forms.
All data collected were loaded to the Review Manager software and checked. The following information was recorded:
a. Publication status and year of publication.
b. Study design and duration of the follow-up.
c. Participants’ features: sample size, with at least 20 teeth, with ≥ five sites of PPD ≥ 5 mm.
d. Methodology of the trials: inclusion and exclusion criteria, randomization, conclusions, and clinical variables.
e. Characteristics of the interventions and control.
f. Source of funding and conflicts of interest.
After reading the title or abstract, 137 articles were excluded (inter-reader agreement k = 0.944}0.056; the following website was used for kappa calculation: https://www.graphpad.com/quickcalcs/kappa1.cfm). When the authors (VR and PO) disagreed in selecting an article for exclusion, judgment by a third author (PM) was solicited.
All authors of the included studies were contacted and asked if they had more information or unpublished material. Just one of the authors replied.10
The full-text reports of the remaining ten articles led to the exclusion of three due to not meeting the inclusion criteria,14 as they included patients with PPD <4 mm,15 smokers, and also administered chlorohexidine in the test group.16
The initial screening of titles and abstracts, in the Lilac database, resulted in two papers for full-text analysis. The two full-text reports were excluded due to not fitting the scope of this work. One of the articles8 was obtained by manual search.
All of the selected studies were randomized clinical trials with a 12-month follow-up. Four studies8-12 evaluated the application of 0.8% HA combined with SRP and three articles2,11,13evaluated the application of 0.2% HA combined with SRP. Most studies suggested that a combined treatment composed of full-mouth SRP and topical administration of HA promoted periodontal healing in CP.8-11,13
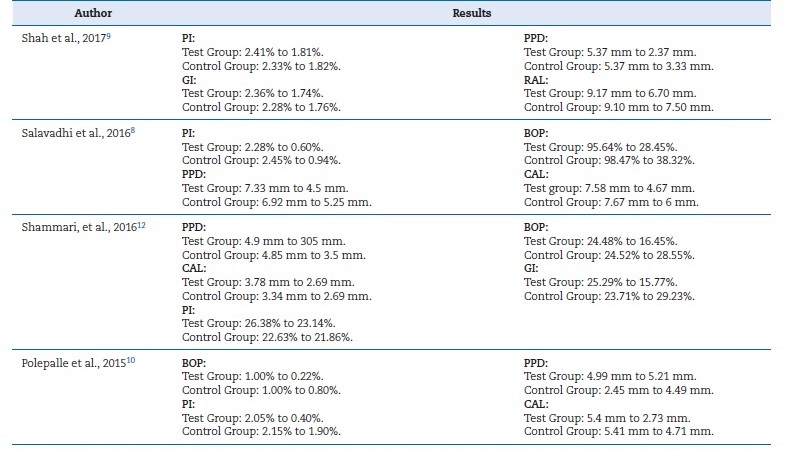
Shah et al.9 detected a significant reduction in PI and GI in both a HA group and a control group at 12 weeks (p<0.05). It also observed a significant reduction in PPD and an increase in RAL in both groups compared to baseline (p<0.05). Compared to the control, the HA group demonstrated a statistically significant reduction in PPD and an increase in RAL (p<0.05) at 12 weeks (Table 1). PI, GI, PPD, and CAL had also improved at 12 weeks (p<0.05) in both groups. Clinically, all indices except CAL had a more statistically significant reduction in tests than controls at 12 weeks. There were statistically significant differences between tests and controls at 12 weeks for GI. For PI, no significant differences were found between tests and controls at 12 weeks, with p-values >0.05 (Table 1). PPD at 12 weeks had lower significant levels in tests than controls, at p=0.4475 with 95% CI (0.0715, 0.8234). Regarding CAL, no significant differences were found between control and test sites for all time points.11
PI - plaque index; GI - gingival index; BOP - bleeding on probing; PPD - probing depth; CAL - clinical attachment level; RAL - residual clinical
attachment
Salavadhi et al.8 observed statistically significant differences in all evaluated parameters between the test and control groups.
At 12 weeks, the differences observed in the test and control groups were related to PI and BOP, which were significantly reduced in the test group. Regarding CAL and PPD, there were also more improvements in the test group than the control.
The results of another trial10 concluded that the application of 0.2 ml of a 0.8% HA subgingival gel for 1 week after root planning in the premolars and canines resulted in a significant reduction of BOP, PI, and PPD (Table 1). On the other hand, the three articles2,11,13that evaluated the application of 0.2% HA combined with SRP indicated it improved the GI and BOP when compared with treatment with SRP alone (Table 2).2,11
GI - gingival index; BOP - bleeding on probing; PPD - probing depth; CAL - clinical attachment level; RAL - residual clinical attachment; Aa - Aggregatibacter
Actinomycetemcomitans; Pg - Porphyromonas Gingivalis; Tf - Tannerella Forsythia
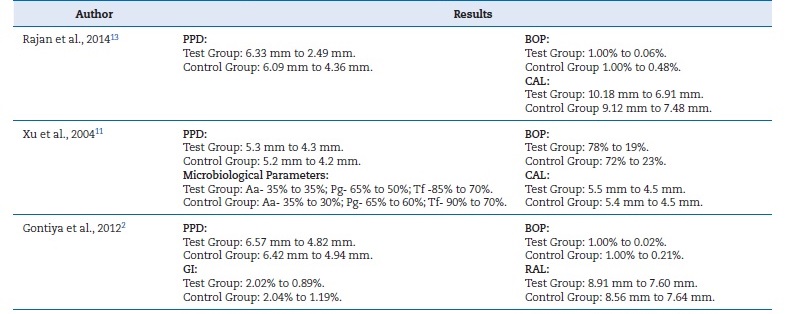
Rajan et al.13 showed that the use of HA as an adjunct to SRP benefited the periodontium health. All clinical parameters evaluated presented improvements compared to the control group. A greater reduction of BOP was observed in the test group. At 12 weeks, PPD showed a statistically significant reduction in the test group from 6.33}0.09 to 2.49}0.51, and in the control group from 6.09}1.26 to 4.36}1.29.13
On the other hand, Xu et al.11detected improvements in all clinical variables (p<0.05) in both groups, but with no clinical differences between tests and controls. It also found no differences between tests and controls in the tested microorganisms.11
Discussion
The aim of this systematic review of the literature was to evaluate the clinical effects of 0.8% or 0.2% HA when used subgingivally as an adjunct to SRP in patients with CP.
HA is a natural component of our body and is abundant is the periodontal tissue; thus, it can be used in humans without causing an immune or inflammatory response. It has viscoelastic properties, which allow it to act as a barrier against periodontal pathogens and make it easier to be used in the form of a gel, with easy application.5
Additionally, it has a pro-angiogenic, anti-inflammatory, and bacteriostatic role, which positively influences periodontal inflammation and healing when used alone or as an adjunct to periodontal mechanical therapy.5 Indeed, most of the studies exposed in this literature review confirm that the use of HA as an adjuvant to scaling seems to obtain better results in the treatment of periodontitis than scaling alone.8-11,13
Side effects of systemic antimicrobial therapy and possible poor compliance of the patient can be minimized by using locally applied antimicrobials. Therefore, a positive influence on the subgingival microbiota can be achieved with locally applied antimicrobials.8
Most of the studies showed a positive effect of HA application together with SRP on the clinical parameters evaluated.8-11,13 No adverse effects have been observed in its application.
The use of HA in the non-surgical treatment of CP relies on its potential impact on the pathological onset, as well as its ability to improve wound healing, due to its previously demonstrated antimicrobial and anti-inflammatory properties,17,18and its pro-angiogenic19,20 21 and osteoinductive potential.18,20
HA has been demonstrated to have a bacteriostatic action on periodontal pathogens when they are in the planktonic phase.17,18However, HA has a lower bacteriostatic potential than chlorhexidine (CHX).18,22
All the included studies compared the outcomes between HA gel application after SRP and the application of a placebo after SRP. Rajan et al.13 detected clinical improvements in the HA group, such as a significant improvement in PPD and BOP and an improvement in CAL. Gontiya and Galgali2 showed a reduction of the gingival parameters in the HA group. It also analyzed the inflammatory infiltrate but did not found any significant differences between the two groups.2 Conversely, Polepalle et al.10 found a significant difference in the inflammatory infiltrate between the HA group and the placebo group. It also noticed a significant improvement in BOP and PPD in the HA group.10 On the other hand, Xu et al.11 did not find improvements in periodontal health following the use of HA as a complement to SRP (without any clinical or microbiological upgrading). However, in this study, the HA gel was applied only once a week for 6 weeks, which is not enough considering the recommended application regimen of three times per day for at least 4 to 8 weeks.
It is important to note that local application treatments are more effective because of their ability to provide localized high concentrations; however, they also have the disadvantage of gingival fluid clearance.23
The included studies do not provide full information on participants. Also, all studies had a follow-up of 12 months, which is relatively short, and, in some studies, the participants applied the products at home. Most studies of this systematic review, after the risk of bias assessment, were classified as having a high risk of bias, according to the Cochrane Collaboration guidelines.24
Conclusions
The data obtained from this systematic review suggest that HA can play a positive role in tissue repair and wound healing.
In periodontitis, the HA + SRP combined treatment offers more satisfactory results than the conventional treatment of SRP alone in most of the outcome variables presented. The findings suggest that the use of HA as an adjuvant should be considered in periodontal therapy.
However, studies still need to be carried out to specify the methods of administration and establish proper recommendations in order to simplify its use.