INTRODUCTION
Antiretroviral therapy (ART) emerged in the 1990s to treat people living with HIV (PLWH). Over the years, mortality and morbidity had a significant decline (Palella et al., 2006). Thereby, HIV infection could be considered a chronic and manageable disease (Erlandson, Schrack, Jankowski, Brown, & Campbell, 2014). Nevertheless, although ART is indispensable for PLHIV, treatment can cause several adverse effects, which can cause interference in physiological systems, with several changes reported in the literature, such as fatigue, insomnia, psychological changes, anaemia, dyslipidemia, lipodystrophy, and cardiovascular problems (Awi & Teow, 2018; Oguntibeju, 2012).
PLWH have higher cardiovascular risk (Jaggers et al., 2013) and abnormalities in the autonomic nervous system (ANS) (Borges, Soares, & Farinatti, 2012; Lebech et al., 2007). At the same time, PLWH have autonomic dysfunction, with decreased heart rate variability (HRV), which may be related to an increased risk of cardiovascular diseases (Borges et al., 2012; McIntosh, 2016). There seems to be a relationship between infection and ART in autonomic dysfunction (McIntosh, 2016; Neild, Amadi, Ponikowski, Coats, & Gazzard, 2000), increasing the sympathetic nervous activity with reduced vagal responses (Lebech et al., 2007; Spierer et al., 2007). Despite the growing interest in HIV/aids and exercise, little is known about the causes of ANS changes, especially during and after exercise (Borges et al., 2012).
Correia et al. (2006) assessed rest using tests such as facial cooling (cold face test) and tilt test. Some studies have already analysed the effects of immersion at different temperatures in terms of physiological responses (Eimonte et al., 2021; Gerrett, Alkemade, & Daanen, 2021; Júnior et al., 2020), performance (Malta, Dutra, Broatch, Bishop, & Zagatto, 2021) and post-effort recovery (Moore et al., 2022). On the other hand, some studies (Costa e Silva, Rodrigues da Conceição, Herdy, Silveira, & Di Masi, 2019; Dionne et al., 2018; Perini, Milesi, Biancardi, Pendergast, & Veicsteinas, 1998) verify differentiated HRV responses during water immersion, observing greater vagal and anti-arrhythmogenic predominance, being beneficial for people with cardiac problems (Dionne et al., 2018).
Considering water immersion as a vagal stimulus, it is opportune to investigate its effects on HRV in PLHIV in rest, during exercise, and post-exercise recovery for a safer prescription of these exercises, with a great clinical contribution. However, no studies are available in the literature that evaluated the effect of water immersion on the autonomic function of PLWH in ART, indicating a gap in the literature. The present study is the first to investigate this effect.
Thus, the present study aims to compare the HRV of PLWH in antiretroviral therapy (ART) with non-infected individuals at rest, during moderate exercise and post-exercise recovery in aquatic and dryland environments. Our initial hypothesis is that water immersion can increase vagal modulation, reducing HR. In this sense, it is expected that the cardiac responses between the groups (PLWH vs non-infected individuals) may be different due to the autonomic dysfunction of individuals PLWH.
METHODS
Study design
The present study is characterised as cross-sectional experimental research (Thomas, Nelson, & Silverman, 2009).
Participants
Twenty (n= 20) participants voluntarily participated in the study. The experimental group (EG) was composed of 10 PLWH (37± 9.6 years) randomly selected from the ambulatory patients’ population in Hospital Graffe & Guille Universidade of the Universidade Federal do Estado do Rio de Janeiro (UNIRIO), Brazil. The control group (CG) was composed of ten non-infected participants (37± 9.5 years), consisting preferably of people in your social life (family and friends). The sample size was calculated using the G * Power 3.1 software. Based on a post-hoc analysis, we adopted a power of 0.80, α= 0.05, correlation coefficient of 0.5, correction non-sphericity of 1 and effect size of 0.38. This analysis of statistical power was performed to reduce the probability of type II error and to determine the minimum number of participants required for this investigation. We found that the sample size was sufficient to provide more than 95% statistical power.
The research project was carried out following the recommendations of the National Health Council (resolution 466/12). We declare that all participants read and signed an informed consent form for study participation, and this study was approved by the Research Ethics Committee (CAAE 37278114.4.0000.5285). The study has no conflict of interest.
The selection of participants was carried out through the analysis of medical records, where inclusion or exclusion criteria were applied. All individuals had received ART for at least six months, were sedentary and asymptomatic. A draw was carried out from the selected medical records to define the chosen ones. The descriptive characteristics of the sample are presented below (Table 1).
Table 1 Sample descriptive characteristics.
| Variables | EG (n= 10) |
CG (n= 10) |
|---|---|---|
| Age (years) | 37± 9.6 | 37± 9.5 |
| Mass (kg) | 79.4± 15.8 | 79.4± 11.2 |
| High (cm) | 173.4± 4.1 | 173.0± 5.8 |
| IT (years) | 9.5± 6.6 | - |
| tART (years) | 8.6± 6 | - |
| CD4+ (cell/mm3) | 724.2± 283.6 | - |
IF: infection time; tART: time of ART; CD4+: CD4 T lymphocyte cells; CG: control group; EG: experimental group.
After selection and preliminary procedures, the participants had anthropometric measurements and familiarisation with the BORG effort perception scale 0-10 (EPS) (Borg, 1982). Participants were allocated to the tests in a randomised cross-over design (all participants were submitted to the exact situations in dryland and aquatic environment).
Procedures and instruments
The tests in the terrestrial environment all took place in the morning in a quiet place, with controlled temperature (between 22 and 24°C) and relative humidity (between 60 and 70%). The heart rate (HR) and HRV were accomplished in the supine position in a bed for 15 continuous minutes, of which only the last 10 minutes were used in the analysis (RESland).
Subsequently, the participants were positioned on a stationary bicycle (Wellness Equipment Center, model Comp., Brasil) to perform the exercise protocol (EXEland). They were instructed to cycle for 20 minutes frequently without any interval, of which 5 minutes with EPS= 9 ("very light" perception); 5 minutes with EPE= 11 ("light" perception); 5 minutes with EPS= 13 ("slightly intense" perception) and 5 minutes with EPS= 15 ("intense" perception) (Borg, 1982; Kesaniemi et al., 2001).
Therefore, to achieve the perception of effort proposed for each phase of the exercise protocol, the subject was given the possibility of accelerating and / or increasing the load to obtain the determined PSE levels. At the end of the exercise protocol, the individuals returned to the bed for recovery and remained in the supine position for 30 minutes, where HR and HRV were continuously measured during the recovery period (RECland).
The tests in the aquatic environment were carried out in an indoor swimming pool with controlled water temperature between 28 and 30°C, and the applied methods were identical to tests in the terrestrial environment, respecting 72 h interval between sessions. The air temperature and relative humidity in the pool environment were between 25 and 31°C and 50 and 80%, respectively. Then, to rest in water (RESwater), the individuals remained in the supine position on "noodle" type floats, similar to that out of the water, so that the transmitter electrode of the cardiofrequencimeter was above the water surface. The rest, exercise, and recovery times were identical to the dryland procedures.
For the water exercise test (EXEwater), a stationary water bicycle with load regulation was used (Masterbike, Meta®, Brasil). Body depth was adjusted, such as (1) the height in the umbilical scar, (2) tolerance of 2 cm (up and down) (3) the transmitter electrode was not submerged. In the end, the individuals returned to the floats to measure HR and HRV continuously for 30 minutes (RECwater).
HR and HRV
The HR and HRV were measured by cardiofrequencimeter Polar RS 800CX® (Polar ElectroTM, Finland). The data were then exported to the Kubios HRV Analysis software (MATLAB, version 2 beta, Kuopio, Finland) to be filtered. The average artifact correction level was used, accepting a maximum percentage of 5% of artifacts, with the routine of removing trend components by the "Smooth priors" method.
For the study in question, mean data from RR intervals, rMSSD (square root of the mean squared differences of successive R-R intervals), and pNN50 (the percentage of the difference more significant than 50 milliseconds between adjacent R-R intervals) were considered in the time domain. In the frequency domain, the power intensity of the signals between frequencies 0.15-0.40 was considered to measure the modulation of the high-frequency bands (HF) that represent parasympathetic activity (Askgaard et al., 2011; McCraty & Shaffer, 2015).
Statistical analysis
A Shapiro-Wilk test was used to verify the normality of the data involved in the study. A two-way ANOVA was performed to determine differences (intra-group and inter-group) in HRV indexes. The Bonferroni post hoc test was performed when appropriate (significant F interaction). A significance level was set at p< 0.05. All the data were described as mean (
RESULTS
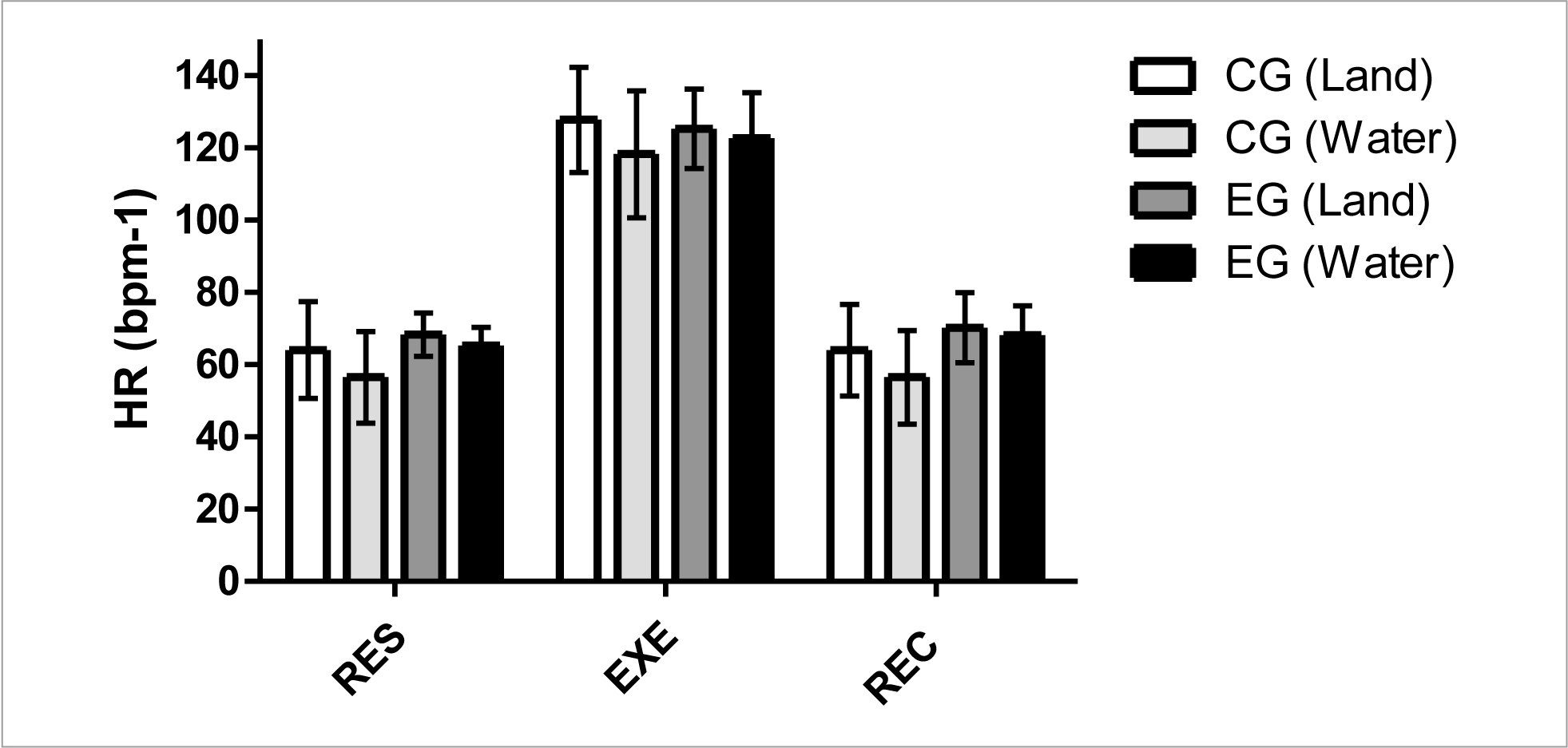
Figure 1 shows HR (bpm-1) results. These data did not indicate a significant difference (p< 0.05) intra- and inter-groups in rest (RES), exercise (EXE), and post-exercise recovery (REC) for situations in aquatic and dryland environments. However, the results showed that water immersion situations generate minors HR mean values compared to dryland situations.
Figure 2 shows the mean R-R interval results. These data indicated a significant difference (p< 0.01) inter-group between CG vs EG in the aquatic environment at RES time. These results indicate that despite water immersion increasing the HRV in rest and post-exercise situations, the values are significantly higher in the non-infected individuals compared to PLWH.
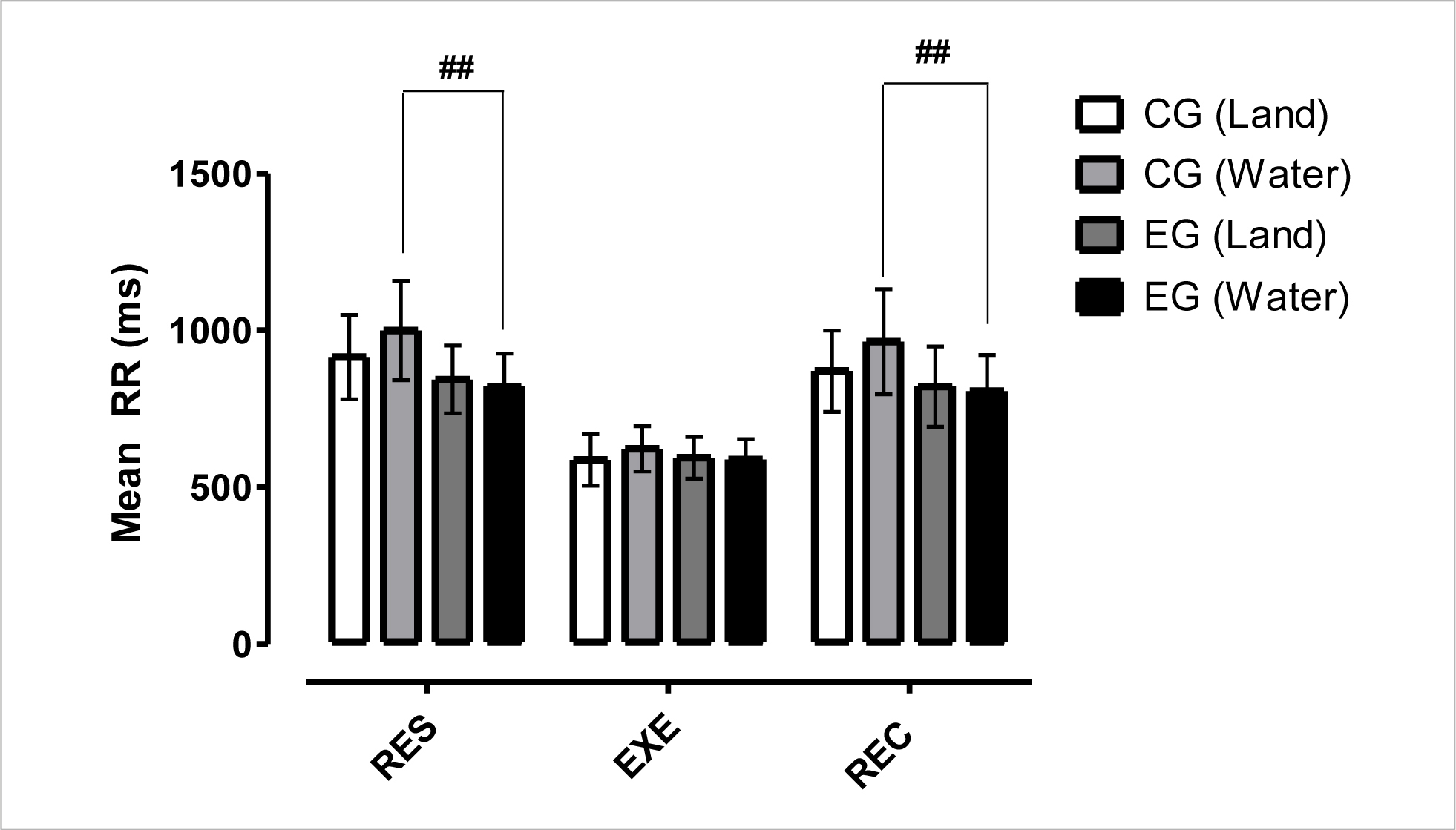
Figure 2 Mean and standard deviation of mean R-R intervals in comparison between different groups and situations.
Figure 3 indicates a significant difference (p< 0.05) between CG vs EG in the dryland environment at RES time, a significant difference (p< 0.001) between CG vs EG in the aquatic environment at RES and REC times, a significant difference (p< 0.01) between CG vs EG (dryland and water) at the REC time. These results point to a significant increase in vagal modulation in rest and post-exercise situations due to water immersion for the non-infected individuals, as well as significant differences for this variable between the non-infected individuals and PLWH.
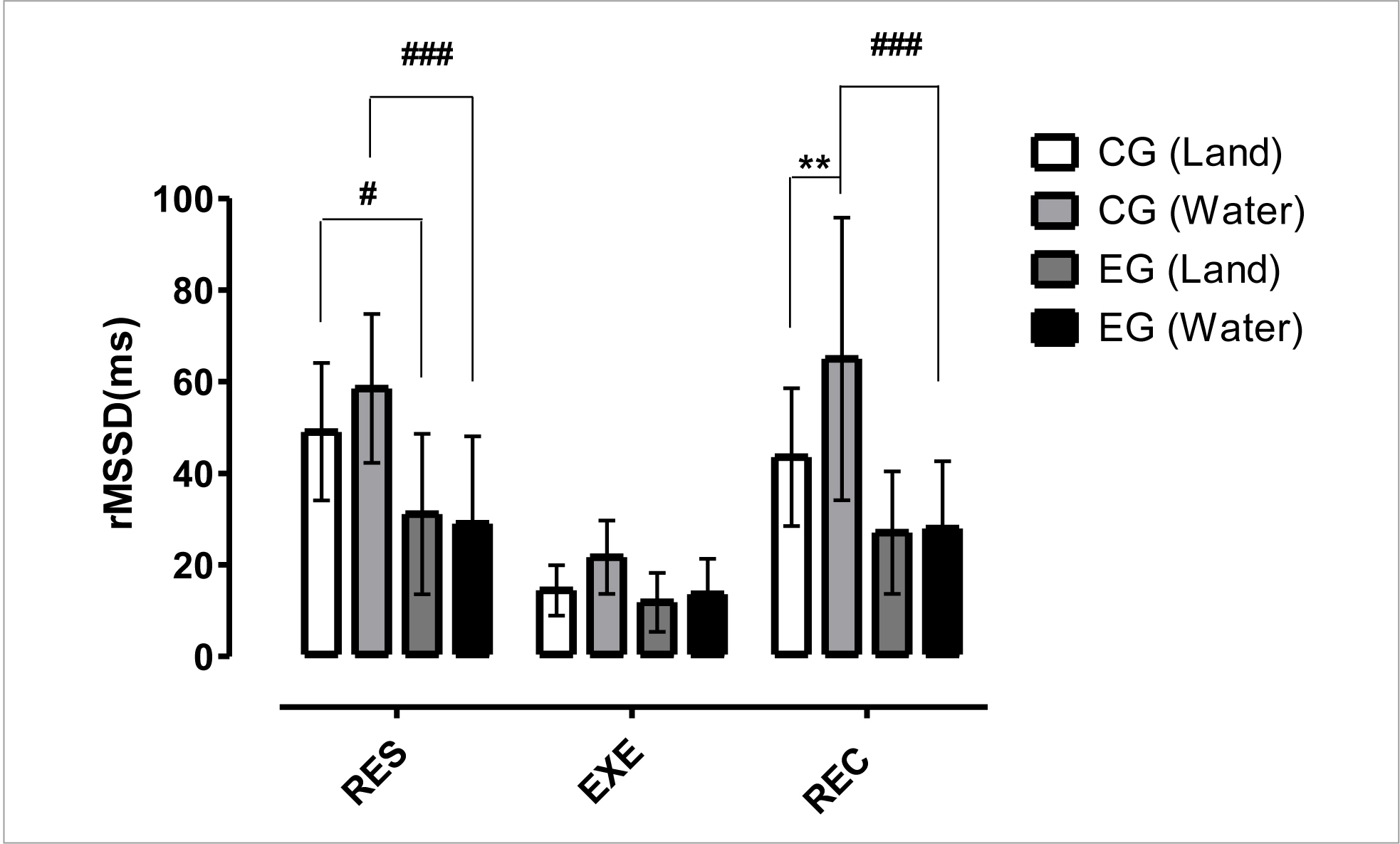
Figure 3 Mean and standard deviation of rMSSD in comparison between different groups and situations.
Figure 4 shows the time domain results for pNN50 (%) index. These data indicated a significant difference (p< 0.05) between CG vs EG in the dryland environment at RES time, a significant difference (p< 0.001) between CG vs EG in the aquatic environment at RES and REC times, a significant difference (p< 0.01) between CGwater x CGland at REC time. These results also showed a significant increase in parasympathetic activity in rest and post-exercise situations due to water immersion for the non-infected individuals, as well as significant differences for this variable between the non-infected individuals and PLWH.
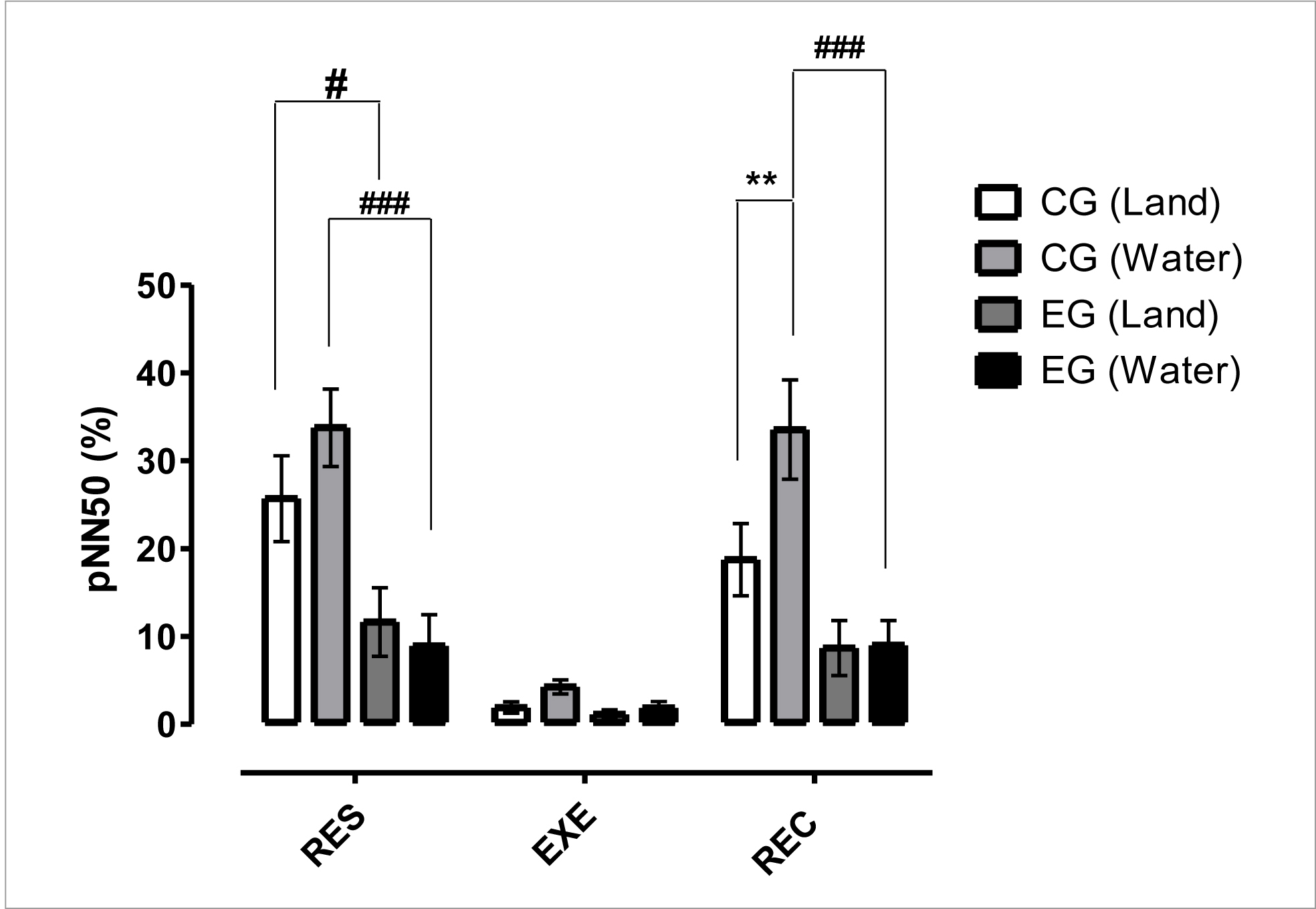
Figure 4 Mean and standard deviation of pNN50(%) in a comparison between different groups and situations.
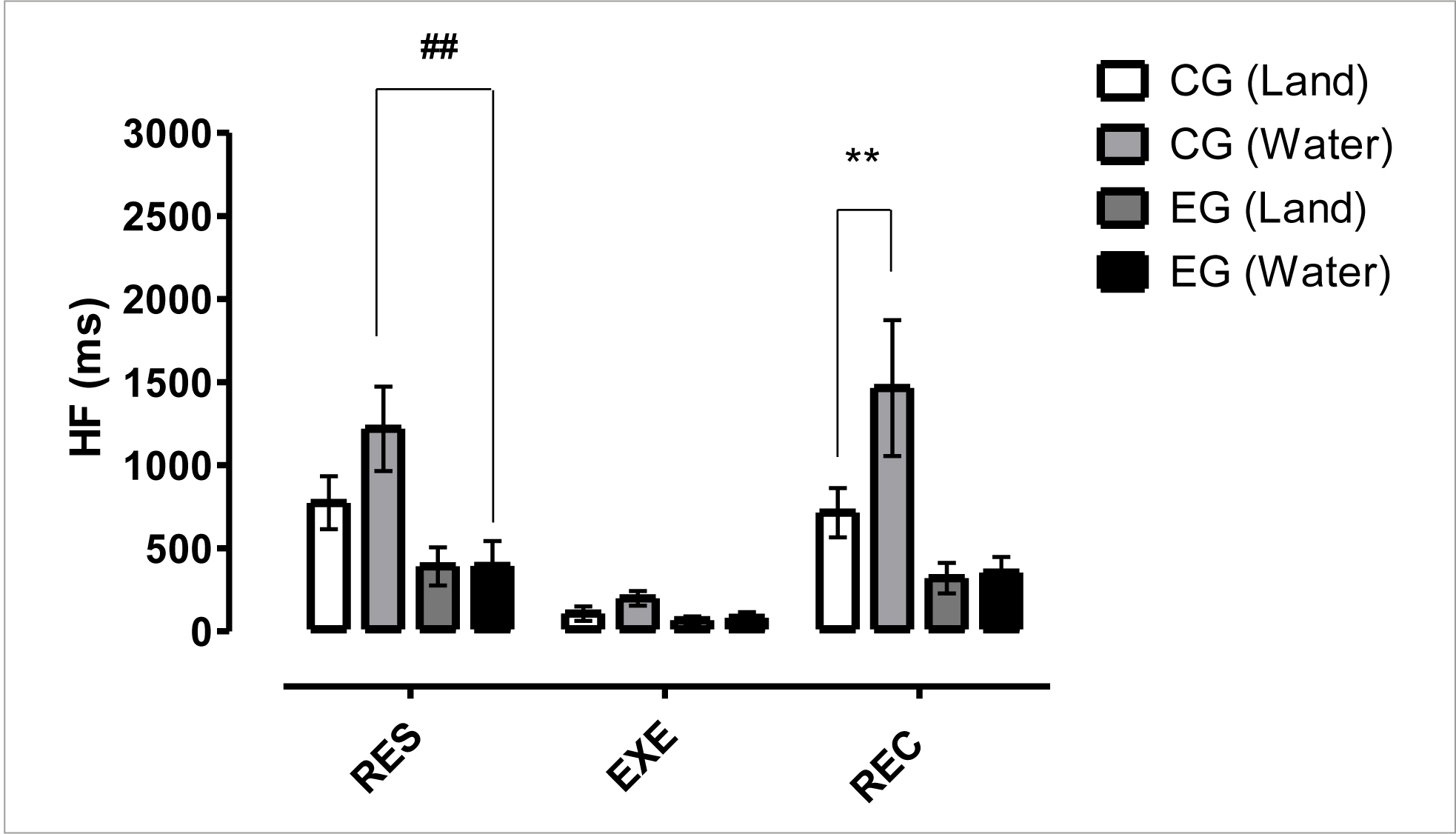
Figure 5 shows the frequency domain results for HF (ms) index. These data indicated a significant difference (p< 0.01) between GC vs EG in the aquatic environment at the RES time and a significant difference (p< 0.01) between CGwater x CGland at REC time. These results showed parasympathetic activity suppression for PLWH in water and dryland, as well as compared to non-infected individuals.
DISCUSSION
The present study aims to compare the HRV of PLWH in antiretroviral therapy (ART) with non-infected individuals at rest, during moderate exercise and post-exercise recovery in aquatic and dryland environments. In this direction, our best results demonstrated that water immersion during rest and post-exercise recovery increases the vagal modulation in non-infected individuals; however, PLWH does not have the same autonomic behaviour, demonstrating that PLWH have autonomic dysfunction.
To better understand the findings, we have divided the discussion into the following topics: HR results, HRV results during exercise, intra-group HRV results in rest and post-exercise recovery, inter-group HRV results in rest and post-exercise recovery and study limitations.
HR results
The intra-group results demonstrated no significant differences at any time (RES, EXE, and REC). However, at all other times, the data showed an HR decrease during water immersion. The observed values are consistent with previous similar studies (Garzon et al., 2015; Yazigi et al., 2013). (Garzon et al., 2015) compared to dryland and aquatic stationary cycling (water at 30°C) at different intensities. The authors did not observe significant differences; however, at all times, the HR was lower in the aquatic environment when compared to dryland. Such results are similar to those found in the present study and corroborated by (Yazigi et al., 2013), where HR was not significantly different between indoor- and water cycling with temperatures between 27 and 31°C.
The inter-group comparative results for CG vs EG in the same moments and environments did not show significant differences. Nevertheless, it is possible to observe slightly higher HR in the EG. In the CG vs EG comparison (dryland environment), the EG showed higher HR in the RES and REC times (4.2 and 6.1 bpm-1). In the CG x EG comparison (water environment), the EG showed higher HR values in the RES, EXE, and REC times (8, 4, and 11 bpm-1, respectively). The decrease can explain these results in a vagal response (Rogstad, Shah, Tesfaladet, Abdullah, & Ahmed-Jushuf, 1999). Evaluating the autonomic responses in PLWH, the authors found an increase in HR, similar to the results normally observed in individuals with high sympathetic activity, as it happens in patients with diabetes mellitus and other diseases.
HRV results during exercise
The HRV results during the 20 minutes exercise time (EXE) did not demonstrate significant differences in one of the intra- or inter-group evaluated parameters. These results were expected because physical exercise generates a vagal withdrawal in function to the exercise intensity (Aubert, Seps, & Beckers, 2003). Even if there were differences, these would have to be analysed with great caution, considering that during dynamic physical exercise, less electrocardiographic signal stationarity and increased participation of non-linear subsystems, as well as the conditions and restrictive premises in the use of spectral analysis are documented as an instrument of mathematical analysis (Marães, 2010).
The exercise results corroborate those obtained by (Borges et al., 2012), who compared HRV in PLWH and controls without finding differences during the exercise, reporting that the intensity of 60% of the VO2 promoted a maximum vagal withdrawal. HRV can be obtained in several physiological conditions. However, it does not always reflect the real changes in vagal and sympathetic activities during exercise due to changes imposed by the adjustment of control mechanisms (Perini & Veicsteinas, 2003).
Intra-group HRV results in rest and post-exercise recovery
The intra-group result at the RES time in the dryland and water comparisons for CG showed significant differences in the rMSSD index; and at the REC time in rMSSD, pNN50, and HF indexes. Considering that rMSDD, pNN50(%) and HF are well accepted to quantify vagal modulation (Laborde, Mosley, & Thayer, 2017), the present results demonstrated that water immersion promotes increases in HRV and better vagal responses in GC when the individual is immersed in a water environment.
The data found in the present study are supported by the literature, such as the results found by (Connelly et al., 1990; Norsk, Bonde-Petersen, & Christensen, 1990). These authors pointed out that when comparing exercise in dryland and aquatic environments, a decreased sympathetic response in the activity inside the water occurs. Stimulation of low and high-pressure baroreceptors by increasing blood in the central body region and increasing cardiac output are possible causes (Florian, Simmons, Chon, Faes, & Shykoff, 2013).
In this sense, the comparative results of dryland vs water in the CG were expected, and there seems to be an advantage in carrying out recovery in the water environment since the decrease in HRV may be associated with pro-arrhythmogenic electrophysiological instability factors, and the parasympathetic activity confers relative electrophysiological stability to the heart (Porto, 2007). Despite advances in research, this premise cannot be affirmed since a series of combined effects and reflexes of immersion remains unknown. The intra-group comparisons (dryland vs water) in the EG did not show differences in any time or environment.
The HRV response during whole-body or partial immersion had yet not been evaluated for this specific population, with no parameter for comparison. A specific test to assess autonomic function involves similar effects of immersion, the cold face test. The cold face test uses an ice pack or ice water for a certain period in order to assess autonomic function by activating the trigeminal nerve. Some studies (Correia et al., 2006; Rogstad et al., 1999) have applied this technique to assess autonomic dysfunction in PLWH, finding not attractive results for PLWH, which suggests dysautonomia for this population. Therefore, our results allow us to speculate that the EG does not benefit from the immersion response, reinforcing the findings of a possible decrease in the vagal response in PLWH.
However, although our study did not find an increase in vagal modulation in PLWH, we observed no reduction, suggesting that there was no greater cardiac overload compared to land-based situations.
Inter-group HRV results in rest and post-exercise recovery
The inter-group results (GC vs EG) at RES time in dryland environment showed significant differences in the rMSSD, pNN50(%) and SD1 indexes, demonstrating a better vagal response (Laborde et al., 2017) in the 10-minute test in the supine position for the CG compared to the EG. The inter-group results (CG vs EG) at the RES time in the aquatic environment showed significant differences in the men R-R intervals, rMSSD, pNN50(%), HF, and SD1 indexes. At the REC time in the aquatic environment, there was a significant difference in the mean R-R intervals, rMSSD, and pNN50(%), thus demonstrating a greater balance of autonomic function for the CG than the EG. Concerning results in the dryland environment, the present study finds an echo in the current literature. Other studies also concluded that HRV is reduced in PLWH compared to non-infected individuals (Askgaard et al., 2011; Borges et al., 2012; Lebech et al., 2007; McIntosh, 2016).
Changes in autonomic function are concerns after ART since, in the past, mortality was high, and the chronic effects of the disease were not well evaluated. With the increase in life expectancy, negative changes in the cardiovascular system started to be investigated. It is currently accepted that the disease of the respective treatment may influence the ANS. The mechanism for developing autonomic dysfunction in PLWH is still unclear, but some hypotheses have been raised. The virus has a predilection for the central nervous system, with a high concentration in the hippocampus, basal ganglia, and other regions involved in the regulation of the hypothalamus. Protease Inhibitors (IPs), specifically Indinavir, have been shown to block GLUT-4 transporters, which appear to be expressed in neurons located in the hypothalamic nucleus. These transporters could be involved in the glucose/insulin detection mechanism in nervous metabolism regulation (Chow et al., 2012). IPs can also directly affect ANS since protease-activated receptors (PARs) are expressed in the brain. Types such as PAR-2 and PAR-3 are found in greater quantities in the thalamus and hypothalamus. Such a location in the brain can predispose these regions to be affected by PIs (Striggow et al., 2001).
There are doubts about the role of ART and HIV infection in isolation. This question is pertinent since the chronic nature of the disease may reveal new adverse effects due to medication and/or greater exposure at the time of infection, a fact that is not yet clear only by infection by the virus or its association with ART (Borges & Farinatti, 2011). As the vast majority of PLWH use the treatment program, isolating analysis of the effects is not an easy task. In this study, the meantime of infection was 9.5 years, and the use of ART was 8.6 years. The mean level of TCD4 was 742 cells/mm3, and viral load was undetectable, being possible to observe adverse effects on the autonomic function in PLWH.
In inter-group comparisons, there were similar results between dryland and aquatic situation. However, it is possible to observe results with high significance, denoting an important immersion response for the CG (not observed in the EG). The data reinforce the findings in the intra-group comparison, in which the EG did not obtain any difference between the mean values.
Corroborating our data, some studies (Bastos et al., 2012; Perini et al., 1998; Perini & Veicsteinas, 2003) have shown HRV differences in the aquatic environment in seronegative individuals, with an increase in vagal response during immersion. The water temperature can also influence the HRV response during immersion, with cold water being considered the best strategy for post-exercise recovery (Almeida et al., 2016). A study conducted by our group found a higher HRV in university students in cold water (22°C) compared to dryland environment in the resting situation (Costa e Silva et al., 2019).
Study limitations
Finally, some factors are limiting for inferences and deserve to be highlighted: the water temperature is relatively warm, the intensity was not controlled by an objective parameter based on percentages obtained by tests of maximum or sub-maximum effort, biochemical variables were not measured, as well as the level of physical activity. Other issues, such as sleep control and food, were not measured. Thus, new experiments with greater control of these factors are suggested, as well as using other types of exercises, sex, and other age groups.
CONCLUSIONS
Water immersion during rest and post-exercise recovery increase the parasympathetic response in seronegative subjects. However, PLWH in ART does not have the same autonomic behaviour, demonstrating that PLWH have autonomic dysfunction compared to controls of the same age. Although the present experiment does not verify an increase in vagal modulation in PLWH during immersion, aquatic activities can be good alternatives to these individuals because immersion situations have not been shown to generate more significant cardiac overload compared to dryland environment situations. Thus, exercises involving water immersion should be prescribed to PLWH as a safe strategy with great clinical potential.
This study is the first to evaluate and compare the effects of exercise and recovery in dryland and aquatic environments with PLWHIV, more research on the subject is recommended.