INTRODUCTION
Humans are born with a natural capacity to learn by integration with environmental stimuli in responses generated by integration with sensory inputs and motor outputs (Hindmarch, 2014). Reflex is the simplest response, and integration can be quite complex, involving Central Nervous System and generating a motor reaction: a psychomotor response. Psychomotor function comprehends physical movement (motor) and cognitive processes. Measurement occurs by accuracy or speed (latency or reaction time) to measure psychomotor performance (Hatfield et al., 2004; Kovaleva et al., 2012).
One of the most evident ways to present psychomotor performance is through sports activity. Movement is necessarily efficient (Hatfield et al., 2004), i.e., it has a low response cost, maxim accuracy, and minimum latency (O’Dwyer & Neilson, 2000; Hatfield & Hillman, 2001). As a result, improving psychomotor performance in athletes is the objective (Hatfield & Hillman, 2001). And to achieve that, learning techniques, psychological techniques, training, nutritional alterations, environmental manipulations, and drugs are used. Sports performance results from both genetic factors and the individual’s degree of experience (Davids & Baker, 2007). The athlete’s performance level depends on his/her morphofunctional characteristics, specific sports demands, and individual experiences in training and competition (Shyamali Kaushalya et al., 2022). Some specific psychomotor skills may be stimulated in athletes at the same time as his/her physical preparation to expand general physical fitness. Transcranial direct current stimulation (tDCS) emerges as a neuromodulation tool to modulate human performance in exercise and sports. Stimulation with this method is done in two ways: the anodal tDCS, considered to stimulate neural areas; and the cathodal tDCS, normally considered as a way to inhibit brain area activity (Brückner & Kammer, 2017).
The tDCS contributed to reinforcing the brain’s important role in regulating exercise performance, integrating physiological and psychological cues (Campos et al., 2016).
Transcranial direct current stimulation (tDCS) is a non-invasive brain stimulation technique through two electrodes that induce alterations in the polarisation of cortical neurons in resting membranes (Nitsche & Paulus, 2000; Machado et al., 2019). Therapeutic use extends to pain control, adjunctive therapy to psychological and neurological pathologies, for example, anxiety, depression, Parkinson Disease, and panic (Lefaucheur et al., 2017). Use in sports has been popular since 2013 (Lefaucheur et al., 2017), improving psychomotor performance by self-stimulation. The literature on this topic is unclear, with positive results in some sports but not in others (Lefaucheur et al., 2017).
The use of tDCS induces changes in skills related to psychomotor performance, such as reaction time, accuracy (motor skill acquisition), and fatigue reduction (Machado et al., 2019). Some authors think that tDCS is a possible way of non-pharmacological doping and breaks the spirit of sports (Davis, 2013). However, some disagree and say tDCS is not doping because do not contradict WADA recommendations; instead, it might generate ethical questions (Holgado et al., 2019a; Zhu et al., 2019). Literature description related to experimental studies regarding tDCS effect may contribute to answering questions about the use of tDCS in athletic competitions.
Although there are other reviews, their objectives were different: Machado et al. (2019) and Alix-Fages et al. (2019) focused on endurance and strength; Holgado et al. (2019b) pointed out exercises and their indexes in a broad way, not in sports; Lattari et al. (2018) concentrated on women; and Shyamali Kaushalya et al. (2022) centred on runners and cyclists. Our review focuses on psychomotor performance, as defined above.
This paper aims to present a systematic review to evaluate the effect of transcranial direct current stimulation on athletes’ psychomotor performance.
METHODS
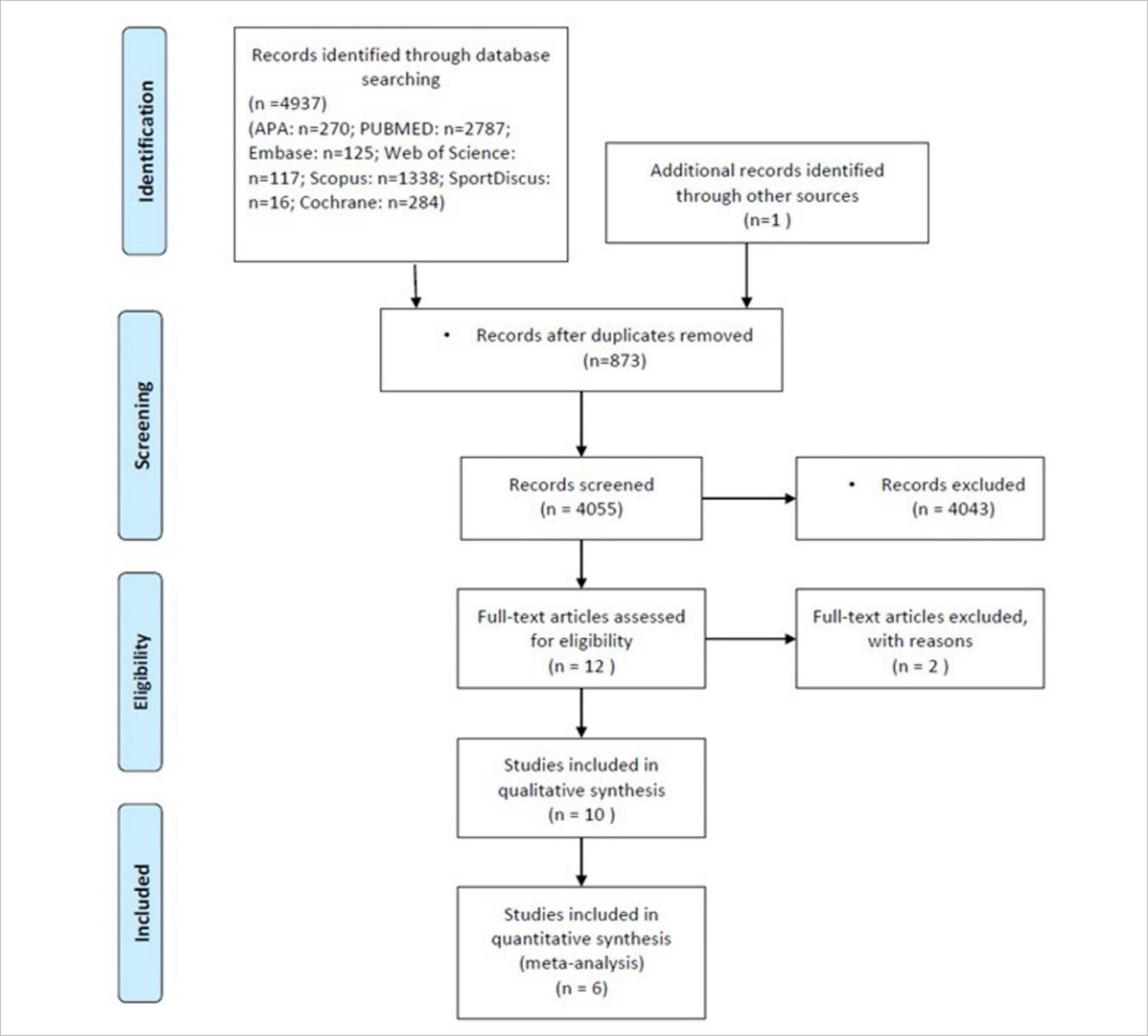
This systematic review and meta-analysis followed the model “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” (PRISMA-P) (Moher et al., 2015) according to Cochrane Manual for Systematic Reviews. We managed citations and references in Mendeley; data were extracted and handled in Excel. This review was enrolled in the international prospective register of systematic review: PROSPERO RD42020210550. Figure 1 summarises this process.
Eligibility criteria
Types of studies
We included experimental studies (randomised controlled trials, cohort, and case-control), cross-sectional and longitudinal studies, and sham-controlled studies reporting Transcranial Direct Current Stimulation effects (tDCS) or High-Definition tDCS (HDCS) on athletes’ psychomotor performance in healthy samples. Dissertations, books, book chapters, reports, conference material, review articles, meta-analyses, instruments’ validation, and scales were excluded from the review.
Types of participants
We considered adults (aged 18+ years), male or female, and athletes who participated in a tDCS study, including psychomotor performance.
Types of outcome measurement
The principal result was related to the functional effect of the stimulation on modulation skills needed for psychomotor performance in motor flexibility, force, and efficacy in sports.
Search strategies
These electronic databases were searched: PsycINFO, PubMed (central), Scopus, Web of Science, Embase, SPORTDiscus, and the Cochrane Library. Empirical studies published in English, Spanish, and Portuguese from January 2009 onwards and whose primary results presented a measurable effect of transcranial direct current stimulation on the psychomotor performance of adult athletes were included.
Search criteria
We selected studies purposing to measure tDCS effect only, using these keywords: (1)’Transcranial direct current stimulation’; (2) ‘tDCS’; (3) ‘HDCS’; (4) ‘Electric Stimulation Therapy’; (5) ‘Neuromodulation’; AND; c) concerning sports: (6) ‘Sports’; (7) ‘Athletic Performance’; (8) ‘Psychomotor Performance’. In addition, we made a search with boolean terms: [(‘Transcranial direct current stimulation’) OR (tDCS) OR (HDCS) OR (‘Electric Stimulation Therapy’) OR (‘Neuromodulation’)] AND [(‘Sports’) OR (‘Athletic Performance’) OR (‘Psychomotor Performance’)].
Selection process
All search results were imported into Mendeley software to manage data and eliminate duplicates. Two independent reviewers made a preliminary title and summaries texts selection for inclusion and exclusion. Subsequently, the full-text selection was made, and two reviewers applied inclusion and exclusion criteria to identify relevant studies to be included in the systematic review analysis. Discrepancies were solved by consensus with the intervention of a third reviewer.
Data extraction process
Data were organised by a reviewer using a standardised extraction form previously prepared in Excel to collect these variables: (1) metadata (authorship, publication date, etc.); (2) demographic data (sample size in each group, age, sex); (3) types of sports (psychomotor performance measures); (4) characteristics of the tDCS technique: electrode position; current intensity; electrode size; current density (current divided by electrode area); the number of data and (5) methods (randomisation protocol; blind evaluation; number evasion). After extracting data, if needed, reviewers addressed any disagreement by consensus with the third reviewer.
Quality assessment
Indicated which study characteristics were assessed and/or any formal risk of bias/quality assessment tools was used. The ‘risk of bias’ was assessed by two independent reviewers using the Cochrane Risk of Bias (RoB 2.0) tool for Randomised Controlled Trials and the Cochrane Risk of Bias in Non-Randomised Studies - of Interventions (ROBINS-I) tool for non-randomised studies (Sterne et al., 2019).
Data synthesis
This study was a meta-analysis, and statistical analysis was carried out using the R software (version 4.0.0) and the R meta-package (https://cran.r-project.org/web/packages/meta/meta.pdf). Each study calculated effect size (i.e., Cohen’s d) to indicate the difference between distinct stimulation conditions considering training and post-training activities (in case of a project between subjects) or using a Cohen-adjusted formula for testing t paired (in case of a project within-subject). We believe several studies would give small sample sizes. We adjusted the sample size to the bias of small decreases - Hedges’ g (Hedges & Olkin, 2014), interpreted as Cohen’s d. As we were expecting decreased heterogeneity between studies, we calculated averages based on a random-effects model for a population, varying according to study heterogeneity. Heterogeneity significance was mandatory using two tests with Q statistics Cochran’s test (P < 0.05). The bias assessment report was presented using the funnel graph asymmetry test (for example, Begg, Egger test).
Analyses were considered from variable testing. In the case of categorical variables (participant characteristics such as sex, age, and outcome measures), they were based on mixed-effects of meta-analytical categorical tests. In this model, studies within subgroups were combined with a random-effects model, and tests for significant differences between subgroups were conducted with the fixed-effects model. Analysis of continuous variables happened with maximum unrestricted likelihood meta-regression to verify significant relationships between continuous variables, considering the Z value and a corresponding p-value.
RESULTS
Overview
A total of 4,055 unique records were screened, and 10 full texts were assessed for eligibility. All studies about pathologies or clinical uses were eliminated from the analysis, remaining six eligible texts for the meta-analysis. This low quantity of articles represents the way of data presentation. The systematic review covered the period between 2009 and November 2020. Figure 1 summarises the study flow. Table 1 summarises studies data.
Table 1 Summarised data of the papers in systematic revision.
| Study | Design Sample and Groups | Experimental Methods And Stimulation |
Psychomotor Measurement |
|---|---|---|---|
| Harris et al. (2019) | Sham-controlled, randomised-group, paired samples, one blind 73 participants (Golf athletes in 4 groups of 19 participants (frontal, 21.7± 2.8, 6F; M1,21.6± 2.9, 5F; V1:20.5±1.0, 7F; or Sham: 22.0±3.7, 11F) | 1 session 1,5 mA right DLPFC, M1 right; VI or Sham (M1), 5 min after test, 3 conditions Baseline, low pression, and High pression | performance (errors), quiet eye period; Anxiety IAMS; |
| Holgado et al. (2019c) | randomised, sham-controlled, single-blind, within-subject design experiment (cross-over) 39 males cyclists, 27(6.8) years, 70.1 (9.5) KG, 3 conditions anodal, cathodal, and Sham |
3 sessions of self-paced, 2 mA, 20 min, DLPFC | ergometric bike: power output, heart rate, flake test (inhibitory test) SRPE and EEG |
| Huang et al. (2019) | In this triple-blind, randomised, sham-controlled study 9 males (20± 1.2 years), 73.1± 6.5 Kg, practice 3 times/week activity in the lasts 6 months |
2 sessions, 5 days interval 2,2 mA, 20 m min, M1 | ; ergometer bike, 50 rpm, resistance 10% weights/6 sec, with intervals 24 s. peak power output and mean power |
| Kamali et al. (2019a) | sham-controlled, paired samples 17 right-handed participants (9 males, 8 females; age 26 to 33 years, with 2 to 3 years of experience in pistol | 2 sessions, 48 h interval, an experimental group with a session sham and other, tDCS 2 mA, 20 min, CB2 right, session 1: 20 min tDSC. |
shots latency, accuracy); Mirror tracing, dynamic tremor tracing |
| Kamali et al. (2019b) | sham-controlled, paired samples, double-blinded 12 experienced male bodybuilders (aging 18 to 44 years, weight 60-120 kg, with regular activity in the lasts 2 years | 2 sessions, 72 h interval, an experimental group with a session sham and other, tDCS 2 mA, 13 min, M1 and TC | Visual analog scale; Hater hate, rated perception extension, one-repetition maximum. Short-term endurance index, The Cambridge brain sciences cognitive platform. Surface electromyography and prefrontal hemodynamic response |
| Lattari et al. (2020) | sham-controlled, double-blinded, crossover study randomised 10 subjects, 22.7± 3.9 years, classified advanced in strength training (47.8 6 22.7 months of training) (1), practitioners of squatting exercises (43.3 6 25.7 months), | 2mA, 20 min, fp2 area, 3 sessions, anodal, cathodal, or sham with 48-72 h interval | Countermovement Jump Kinematic Test-Retest Reliability, Countermovement Jump Assessment in Experimental Conditions |
| Mesquita et al. (2019) | sham-controlled, paired samples 19 TKD athletes,12 men, 7 women; mean ± SD, age: 19± 3 years; body mass: 60.7± 6.9 kg; height: 171.7± 6.9 cm; body fat: 13± 8%; practice time: 8.9± 5.0 years; level: international/national | athletes were randomly assigned in a single-blind and counterbalanced order to either the anodal (a-tDCS) or the sham condition. In each session, the subjects executed performance assessments composed by CMJs and the FSKT immediately and 1 h after stimulation. Additionally, subjects should report their session rating of perceived exertion (session-RPE) 30 min after the performance assessment. Experimental sessions were performed at the same time of the day and were interspaced by at least 48 h 1.5 mA, 15 min, C3 and C4 | Countermovement Jump. Two minutes after the warm-up the subjects performed 3 CMJs with 1-minute rest between them; Frequency Speed of Kick Test (FSKT)- Time of reaction |
| Okano et al. (2015) | single-blinded 10 subjects, 33± 9 years; national-level cyclist, 10-11 years training. | 2 sessions, 24 h interval, 2mA, 20 min, TC area, anodal T3, | Maximal incremental exercise test, RPE responses |
| Seidel & Ragert (2019) | sham-controlled, crossover, double-blinded 46 participants, male and female divided into 3 groups (football, handball, and non-athletes. 2 years of regular practice and participation in competitions. | 2 sessions, 24 h interval, 2 mA, 20 min, area M1 bilateral, session 1: 20 min tDSC, testing before, in 10 min on stimulation period, after stimulation (0 min and 30 min); session 2: idem | reaction time tasks (RTT) and tapping tasks (TT) |
| Seidel-Marzi & Ragert (2020) | sham-controlled, double-blinded, crossover study. 46 participants, divided into 3 groups (football, handball, and non-athletes. 2 years of regular practice and participation in competitions. 13 FB (three females, mean age= 24.00± 3.89 years), 12 HB (five females, mean age= 22.50± 4.32 years) and 21 NA (11 females, mean age= 26.95± 3.43 years). On average, FB trained for 16.31± 5.02 years and currently 5.65± 2.15 h/week, whereas HB trained for 13.17± 4.49 years and currently 8.54± 3.84 h/week in their respective sports disciplines. On the other hand, NA performed an average of less than 2 h | 2 sessions, 24 h interval, 2 mA, 20 min, M1 bilateral, session 1: 20 min tDSC, testing before, in 10 min on stimulation period, after stimulation (0 min and 30 min); session 2: idem | reaction time tasks (RTT) and tapping tasks (TT) |
Study characteristics
Design
All but one study (Okano et al., 2015) were randomised, and all had some level of blinding: one triple-blinded design and two double-blinded designs. Most studies were crossover, with a sham control in some subjects; however, three of them (Kamali et al., 2019a; Kamali et al., 2019b; Mesquita et al., 2019) had a paired group design.
Sample
50% sample was composed of high-level athletes (Holgado et al., 2019c; Lattari et al., 2020; Mesquita et al., 2019; Seidel & Ragert, 2019; Seidel-Marzi & Ragert, 2020) and 50% by novices or non-professional athletes (Huang et al., 2019; Kamali et al., 2019a; Kamali et al., 2019b; Mesquita et al., 2019; Okano et al., 2015). Type of athletic activity varies, including football and handball (Seidel & Ragert, 2019; Seidel-Marzi & Ragert, 2020), cycling (Holgado et al., 2019c; Huang et al., 2019; Okano et al., 2015), golf (Harris et al., 2019), taekwondo (Mesquita et al., 2019), pistol shot (Kamali et al., 2019a) and bodybuilder (Kamali et al., 2019b; Lattari et al., 2020).
Stimulation
Most studies (60%) used 2 mA by 20 min (Holgado et al., 2019c; Huang et al., 2019; Kamali et al., 2019a; Kamali et al., 2019b; Lattari et al., 2020; Okano et al., 2015; Seidel & Ragert, 2019; Seidel-Marzi & Ragert, 2020). Two studies (20%) used 1,5 mA by 15 min (Harris et al., 2019; Mesquita et al., 2019): one of them (10 %) used 2, 2 mA by 20 min, and the other one (10%) used 2 mA for 13 min (Huang et al., 2019). The stimulation area was diversified, with the presence of M1 bilateral (Seidel & Ragert, 2019; Seidel-Marzi & Ragert, 2020) or unilateral (Huang et al., 2019), CB2 right (Kamali et al., 2019a; Kamali et al., 2019b), C3 and C4 (Mesquita et al., 2019), F4, OZ DLPFC (Harris et al., 2019; Holgado et al., 2019c), FP2 (Lattari et al., 2020), TC and T3 (Okano et al., 2015)
Psychomotor measurement
The measure, quite diverse, was accomplished in time reaction (latency to response) (Kamali et al., 2019a; Lattari et al., 2020; Mesquita et al., 2019; Seidel & Ragert, 2019; Seidel-Marzi & Ragert, 2020), repetition (Kamali et al., 2019a; Mesquita et al., 2019; Okano et al., 2015; Seidel & Ragert 2019; Seidel-Marzi & Ragert, 2020), power (Harris et al., 2019; Holgado et al., 2019c; Huang et al., 2019; Kamali et al., 2019b; Okano et al., 2015), or accuracy (Kamali et al., 2019a). Other non-psychomotor measurements were present in many articles, especially psychological measurements of anxiety and impulsivity, or physiological measurements, i.e., heart rate or EEG (Holgado et al., 2019c).
Results
Positive effects of tDCS were demonstrated in half of the studies (Huang et al., 2019; Kamali et al., 2019a; Kamali et al., 2019b; Okano et al., 2015; Seidel-Marzi & Ragert, 2020). One study analysed negative effects (Mesquita et al., 2019), and four papers presented no effects (Harris et al., 2019; Holgado et al., 2019c; Lattari et al., 2020; Seidel & Ragert, 2019). Power dimension is the most common effect (Huang et al., 2019; Kamali et al., 2019b; Okano et al., 2015; Seidel-Marzi & Ragert, 2020), and one study, with shooters, related effect and accuracy (Kamali et al., 2019a).
The power effect was investigated in objective measures (for example, motor movement’s repetition or power in a cycle) and subjective ones (fatigue test).
Risk of bias
In most of the studies, our study sample had a low risk of bias, except one with a high risk of blinding assessment. Figure 2 presents this analysis.
Meta-analysis
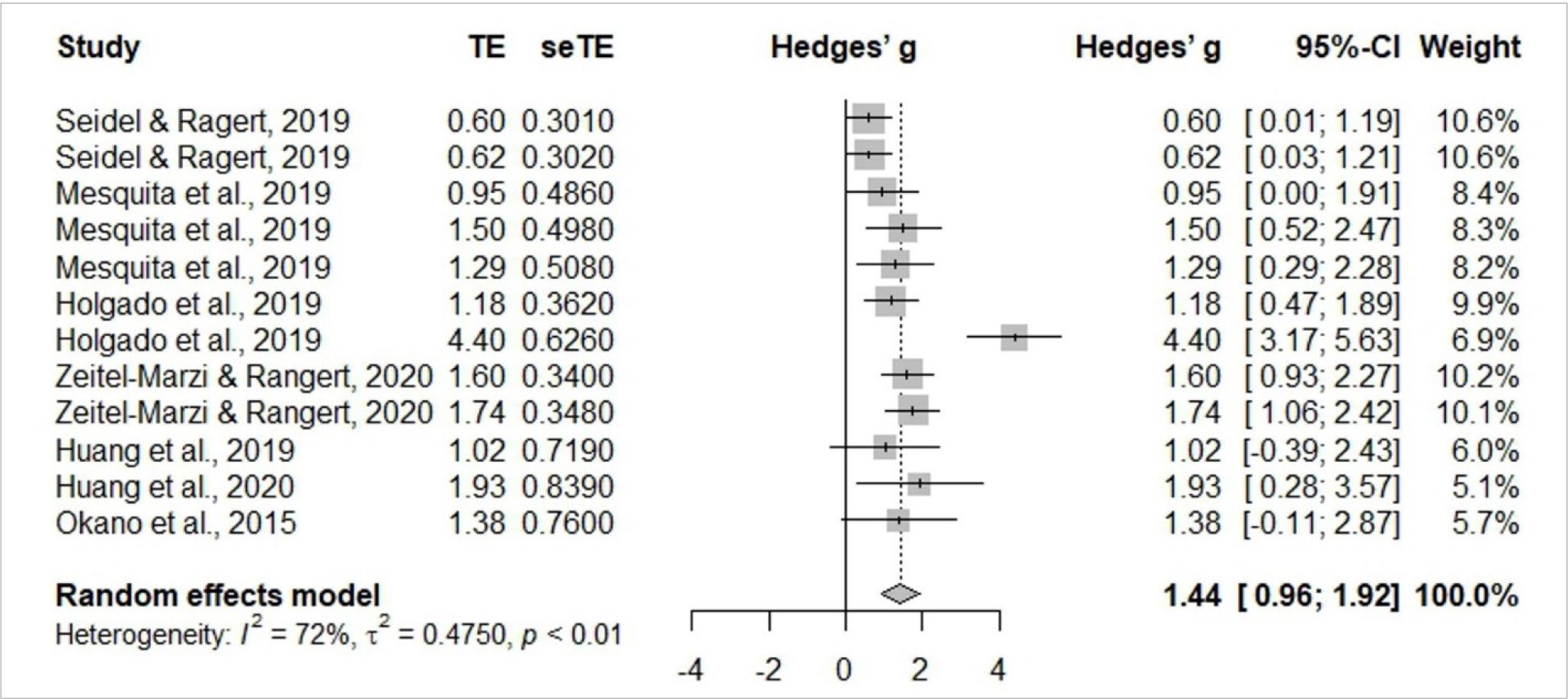
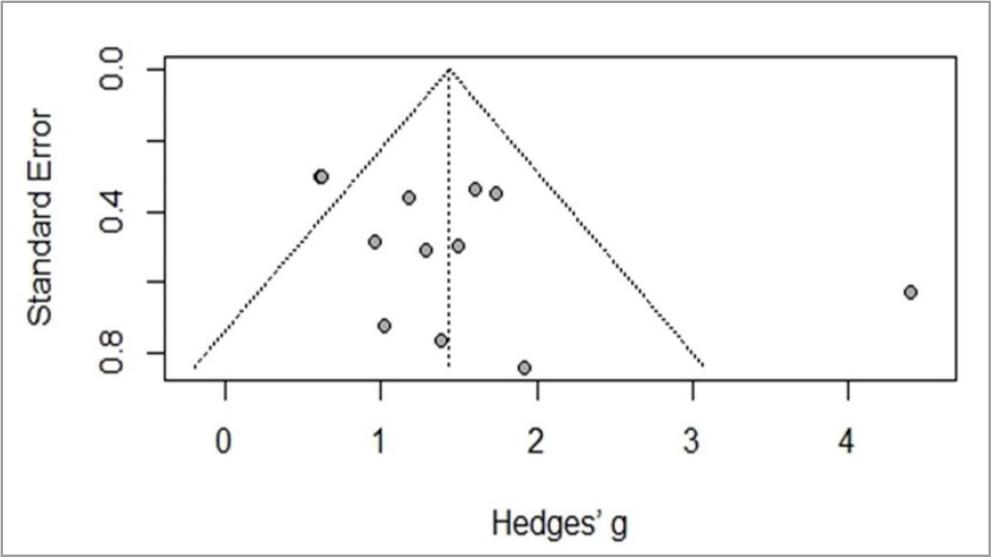
Meta-analysis is identified in Figures 3 and 4, and includes six studies (Holgado et al., 2019c; Huang et al., 2019; Mesquita et al., 2019; Okano et al., 2015; Seidel & Ragert, 2019; Seidel-Marzi & Ragert, 2020). General study meta-analysis identified significant heterogeneity of I2= 72%, t2= 0.4750, p< 0.01, indicating the use of the random effects model is adequate. The results confirmed a significant effect Hedges’ g= 1.44, 95%CI 0.92–1.92. Assessing study publication bias, the funnel graph confirmed a degree of asymmetry. Egger’s test revealed an intercept value of 2.627 [95%CI –0.509– –5.763; t= 1.661] with a p-value of 0.1277, indicating no substantial asymmetry in the funnel graph, so there is no evidence of publication bias.
DISCUSSION
Our analysis identified that tDCS had some effects on the athlete’s psychomotor performance, however, it is a controversial fact. There is no homogeneity in performance dimensions, namely, strength, accuracy, and latency. Analysed articles confirm the effect on force dimension in those who report some effect by direct measure (W or Kg), indirect measure (perceived effort), or repetition speed measure, which can be considered as a force dimension. Even accuracy data, obtained from snipers, seems to indicate power data and can be explained by better control of the weapon’s retro-shot.
Relying on experimental plans and their specific objectives, either stimulated areas or test types and sports are diverse, not allowing a minimal general protocol for the tDCS use in the area. To know if there is a real effect of tDCS in each psychomotor dimension, it would be necessary to describe accurately the existence of each sport and test these activities separately. Thus, it would be required to stimulate different areas and to test the effect of strength, accuracy, and latency (response time) in a laboratory situation. In a laboratory study concerning trained cyclists submitted to anodic tDCS before exercise, Okano et al. (2015) demonstrated improved dynamic motor performance (incremental exercise test) and tolerance to athletes’ physical effort. As for sport applicability, specifically concerning motor fatigue because of exhaustive physical work maintained for a long period, Seidel-Marzi and Ragert (2019) evidenced that regardless of sport’s requirement or athlete’s training level, tDCS can reduce motor fatigue during rapid repetitive movements.
On the other hand, in sports requiring a closed motor skill, such as golf, for example, which performance is particularly important to control visual attention (golf courses at baseline), no beneficial effects were observed after receiving tDCS (Harris et al., 2019), reinforcing evidence of no learning transfer to actual sport performance. In this sense, it would be necessary to consider the specific requirements of each sport to these dimensions and their combinations. Only in this way could we answer if tDCS influences sport so that it can be considered doping or not and if the effect on psychomotricity is of such a magnitude that it can be considered a non-sports intervention.
However, we cannot make a conclusion on the current state of the art reflected in this review. Although the articles have a good experimental design, they do not present data density to support a definitive conclusion or construction of a stimulation protocol for athletes in training or competition.
Our work has several limitations coming from the articles used: on the one hand, there are diverse ways to perform the tDCS; on the other hand, the methods to measure the psychomotor effect vary a lot. Moreover, this has an impact on our review results.
CONCLUSIONS
The tDCS has an effect on strength, but this is not clear and may depend on the sport’s requirements or procedure variations. Nonetheless, currently, it is not possible to define a safe and effective tDCS use protocol for athletes to increase psychomotor performance. Due to this, more parameterised studies are necessary to develop protocols to use in this population, to improve psychomotor activity.