INTRODUCTION
Various treatments have been proposed as part of the search for increased life expectancy and quality of life for HIV-infected patients. The primary pharmacological strategy for HIV treatment is highly active anti-retroviral therapy (HAART). HAART may produce severe side effects such as metabolic syndrome and lipodystrophy (Bittencourt, 2007; Castelo Filho, & Abrão, 2007). Physical exercise has shown to be effective in redistributing adipose tissue (Santos, Pereira, Silva, Lazzarotto, & Petersen, 2013) and improving metabolic syndrome (Lauriola et al., 2010), and it has been recommended as a strategy to be used together with HAART (Brasil, 2008). Furthermore, the use of blood flow restriction (BFR) in HIV-infected patients showed a significant increase in muscle strength and skeletal muscle tissue as much as traditional training (Alves et al., 2020).
Among previously studied methods, Lazzarotto et al. (2010) reported eight studies in which concurrent training with single or multiple series was used and observed that both types of training improved muscle and cardiorespiratory function. Calabrese and LaPerriere (1993) complemented these data, stating that exercises for asymptomatic patients should not be restricted and that such exercises may include high-intensity activities. On the other hand, patients who have shown at least one symptom may exercise but should be careful to avoid exhaustion.
Studies show that high-intensity physical exercise temporarily depresses the immune system, providing an “open window” for the development of infections (for example, upper respiratory tract infections) that may last for up to 72 hours after a training session, whereas exercise of mild to moderate intensity contributes to the maintenance and optimisation of immune responses (Nieman & Nehlsen-Cannarella, 1994). Despite this, some authors have used training loads of 70 to 80% of one-repetition maximum (1 RM) during strength training sessions of HIV patients, a regimen that resulted in chronic improvement of immune markers (Brito et al., 2013; Mendes et al., 2013).
Similarly, BFR has been used in strength training for more than four decades (Sato, Yoshitomi, & Abe, 2005). This method aims to increase muscle mass by combining low-intensity loads (20–40% of 1 RM) with compression of the upper or lower limbs by a sphygmomanometer cuff or a pneumatic tourniquet, thus, causing less mechanical stress. It can be a better method to use with patients on rehabilitation, as in patients with some inflammatory disease (Patterson et al., 2019), like in people living with HIV (Alves et al., 2020). Due to this advantage, testing this method in patients with special conditions, such as HIV-infected patients, has been proposed (Brito et al., 2013). However, the immune and inflammatory mechanisms involved in response to this type of training are complex and are still not completely clear.
About this topic, it is possible to state that after an exercise training session, the leukocyte count tends to decrease after 30 minutes, reaching lower numbers than baseline or pre-exercise levels (Dias, 2009) as a result of redistribution and apoptosis, mainly of neutrophils and lymphocytes (Pedersen et al., 1997; Pedersen & Hoffman-Goetz, 2000; Leandro, Castro, Nascimento, Pithon-Curi, & Curi, 2007; Walsh et al., 2011; Freidenreich & Volek, 2012) with neuroendocrine factors, especially cortisol, being one of the causes of this leukopenia (Pedersen & Hoffman-Goetz, 2000; Malm et al., 2004).
We hypothesized that the BFR training modulates the immune response without promoting immune suppression in people living with HIV. Our aim was to assess the modulation of leukocytes and the immunoinflammatory effects of BFR in HIV patients to permit clarification of the inflammatory and immunosuppression processes involved in this type of training and its relationship to hormonal responses (Pedersen et al., 1997; Leandro et al., 2007; Paulsen, Raastad, & Peake, 2012).
METHODS
Participants
This case study describes the response of segmented neutrophils, monocytes, lymphocytes, and lymphocyte subpopulations (CD4+ T and CD8+ T lymphocytes) to BFR training in two (2) HIV-infected patients. The physically active patient (ACTIV) was 37 years old, and her body mass was 46.6 kg, and her height was 1.58 m, yielding a BMI of 18.67 kg/m2. The patient with a sedentary lifestyle (SED) was 39 years old and BMI= 31.37 kg/m2 (weight= 85.4 kg; height= 1.65 m). The present research was approved by the Research Ethics Committee of the Health Sciences Centre of the Federal University of Paraíba, according to National Health Council Resolution CNS 466/12 (Number: 444.854).
The patients enrolled in this study were female and had clinical conditions that permitted the use of strength training as assessed by the medical team of the HIV/AIDS Specialised Care Service. The exclusion criteria included negative peripheral arterial disease, availability to train, not signing an informed consent form, and use of anti-retroviral therapy.
Blood analysis
Blood (6 mL) was collected from the median cubital vein of each participant before the training session began (Federal), immediately after the training session (post), and 30 minutes after training (30 min). After collection, the blood was transferred to two test tubes containing ethylenediaminetetraacetic acid-EDTA-K3 (0.15% volume/final volume solution). Two millilitres of blood were placed in one test tube to be used for the white blood cell (WBC) count, and 4 mL was transferred to another tube to be used for CD4+ and CD8+ T lymphocyte counts. All biological material was stored in insulated boxes at room temperature (18°–22°C) for a maximum of two hours.
WBC was performed at the Clinical Analysis Division of the Haematology Laboratory, Lauro Wanderley University Hospital, Federal University of Paraíba. Samples were homogenised in a homogeniser for haemogram tubes and subsequently analysed in a Cobas Mira Plus® (Roche Diagnostic System) biochemistry analyser. Lymphocyte subpopulation counts were performed at the Public Health Central Laboratory of the State of Paraíba (Laboratório Central de Saúde Pública do Estado da Paraíba — LACEN-PB) using flow cytometry equipment (FACSCalibur®, Becton Dickinson, San Jose, CA, United States of America) with a coefficient of variation of 3% for the analysis of CD4+ and CD8+ T lymphocytes.
Anthropometric assessment
Body mass was measured with a 100-g precision scale, and height was measured with a 0.1 cm precision stadiometer attached to the scale. Body mass index (BMI) was calculated using the following Equation 1:
Blood flow restriction assessment
Restriction pressure (mmHg) was determined according to Laurentino et al. (2012) using a blood pressure sphygmomanometer (18 cm wide x 80 cm long) placed on the inguinal region of the thigh. A sphygmomanometer with a 12 cm wide x 50 cm long cuff was placed in the proximal area of each arm to determine the occlusion point of the upper limbs. The cuff was inflated until the auscultation pulse of the tibial (for lower limbs) or the radial (for upper limbs) artery was interrupted; the restriction pressure was calculated as 80% of the maximum arterial occlusion pressure (AOP). For vascular assessments, the patients reclined in a supine position for a Doppler exam with portable equipment (DV2001-Medpej). The transducer was placed on the skin with coupling gel towards the trajectory of the dorsal artery of the foot (lower limb point of restriction) and the radial artery (upper limb point of restriction) at a 60° angle.
One-repetition maximum assessment
To determine the percentage of exercise load for each participant, the 1 RM test was performed according to the mathematical model proposed by Baechle and Groove (2000) – Equation 2:
To minimise the test's margin of error, the volunteers received standard instructions prior to the tests to allow them to be aware of all data collection procedures (Dias et al., 2005). Thus, the following procedures were adopted: the patients received information on the technical aspects of exercise execution; the researchers monitored the techniques used by the patients during the exercises; the test began with a warm-up that consisted of four to 10 repetitions at 50% of the load used for the test; after a five-minute break, the exercise was performed at 80 to 100% maximum load; the participants attempted to perform the maximum number of repetitions until concentric failure; if the number of repetitions was greater than ten (10), the load was adjusted, and the participant repeated the exercise after a three- to five-minute break. The participants were inactive for a period of 48 hours prior to the training sessions.
Training sessions
Training sessions started with a warm-up on an exercise bicycle. The resistance exercises were performed at an intensity of 30% of 1 RM with blood flow restriction. Four sets (one set of 30 repetitions and three sets of 15 repeats) totalizing 75 repetitions with a 30-second interval between sets. An interval of 2-minutes between exercises was taken for each exercise. The speed of repetitions was monitored using a digital metronome that provided a rhythm of two seconds in each movement phase (concentric and eccentric). A phase of adaptation preceded the training phase to the exercises and the BFR pressure device. Two strength exercises were performed: one for the muscle groups of the upper body (flat bench press) and another for the lower limbs (knee extension).
Statistical analysis
Descriptive statistics were used to present pre-intervention data. To assess time effects, percentage variations (r%1) in blood tests before and immediately after training (r%1), immediately after and 30 minutes after training (r%2) and before and 30 minutes after training (r%3) are shown. Data showing the effects on patients are presented in graphical form.
RESULTS
The ACTIV had been infected with HIV for more than 10 years and was in an asymptomatic disease stage. This patient had been working out for two months at a frequency of two training sessions per week. Her HAART regimen included Tenofovir, Lamivudine, and efavirenz. Coronary disease risk was predicted by the waist-hip ratio (WHR) as moderate (WHR= 0.78), and peripheral obstructive vascular disease was negative (ABI= 0.96). The result of the submaximal dynamic force test was 22.75 kg for the flat bench press and 50.56 kg for knee extension.
The SED had also been infected with HIV for more than 10 years and was in an asymptomatic disease stage. She was not physically active in her free time. Her HAART regimen included Biovir (lamivudine+ zidovudine) and Kaletra. Coronary disease risk predicted by WHR as very high (WHR= 0.96), and peripheral obstructive vascular disease (ABI= 1.02). The result of the submaximal dynamic force test for flat bench press was 27.00 kg, and for knee extension was 88.00 kg.
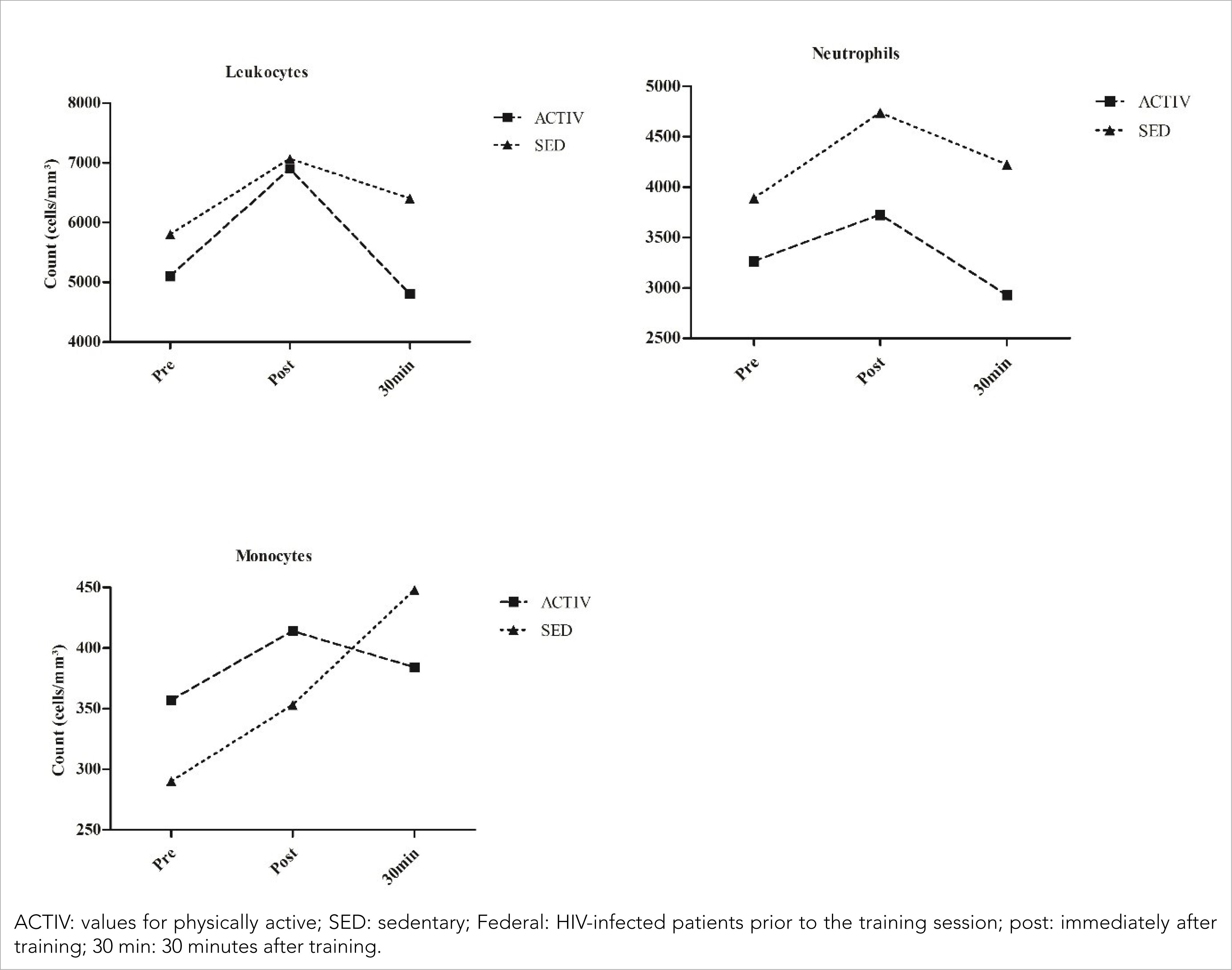
After one BFR training session, leukocyte modulation was observed in both patients (Figure 1) [ACTIV (pre= 5100 cells/mm3; post= 6900 cells/mm3; 30 min= 4800 cells/mm3); SED (pre= 5800 cells/mm3; post= 7069 cells/mm3; 30 min= 6400 cells/mm3)]. In both patients, modulation was primarily due to changes in the number of neutrophils [ACTIV (pre= 3264 cells/mm3; post= 3726 cells/mm3; 30 min= 2928 cells/mm3); SED (pre= 3886 cells/mm3; post= 4736 cells/mm3; 30 min= 4224 cells/mm3)]. Monocytes responded differently in the two patients, showing reduced counts in the ACTIV and increased counts in the sedentary patient [ACTIV (pre= 357 cells/mm3; post= 414 cells/mm3; 30 min= 384 cells/mm3); SED (pre= 290 cells/mm3; post= 353 cells/mm3; 30 min= 448 cells/mm3)].
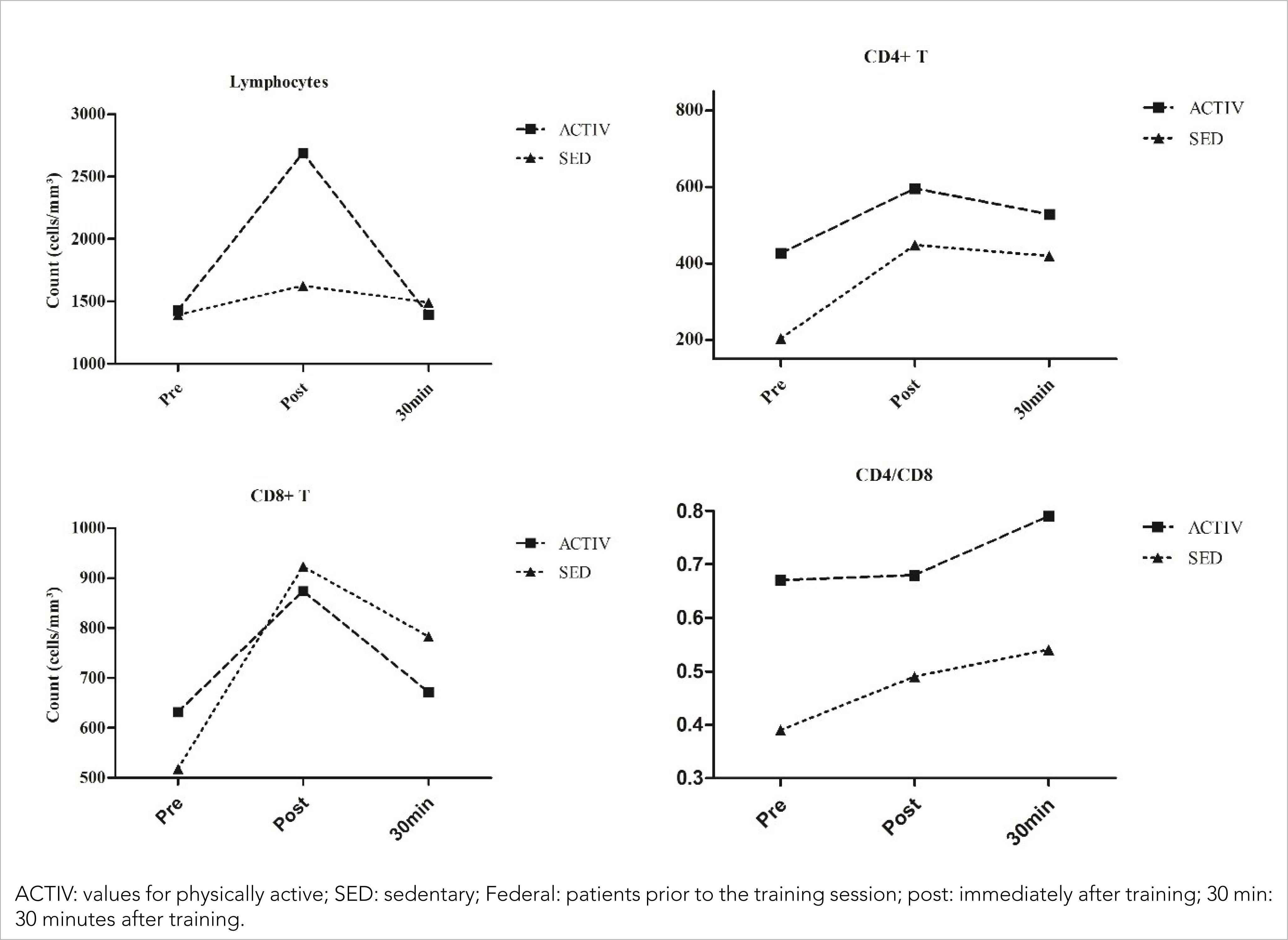
Lymphocyte modulation was more intense in the ACTIVE than in the SED (Figure 2) [ACTIV (pre= 1428 cells/mm3; post= 2691 cells/mm3; 30 min= 1392 cells/mm3); SED (pre= 1392 cells/mm3; 1626 cells/mm3; 30 min= 1492 cells/mm3)]. By studying the modulation occurring in lymphocyte subpopulations, similar variations were found in CD4+ T and CD8+ T cell counts [CD4+ T: ACTIV (426 cells/mm3; post= 596 cells/mm3; 30 min= 528 cells/mm3); SED (pre= 203 cells/mm3; post= 448 cells/mm3; 30 min= 419 cells/mm3); CD8+ T: ACTIV (pre= 632 cells/mm3; post= 874 cells/mm3; 30 min= 672 cells/mm3); SED (pre= 518 cells/mm3; post= 923 cells/mm3; 30 min= 783 cells/mm3). These results showed an increase in the CD4+ / CD8+ T lymphocyte ratio in both patients (pre= 0.67; post= 0.68; 30 min= 0.79); SED (pre= 0.39; post= 0.49; 30 min= 0.54)].
About percentage variations (Δ%) in blood, both patients had an increase when compared the moments before and immediately after training following by a decrease between before and 30 minutes after training, except for monocytes in SED. Leukocytes showed Δ% for ACTIV (Δ%1= 35,29; Δ%2= −30,43; Δ%3= −5,88) and SED (Δ%1= 21,88; Δ%2= −9,46; Δ%3= 10,34), for neutrophils the Δ% was ACTIV (Δ%1= 14,15; Δ%2= −21,42; Δ%3= −10,29) and SED (Δ%1= 21,47; Δ%2= −10,81; Δ%3= 8,70) and monocytes showed ACTIV (Δ%1= 15,97; Δ%2= −7,25; Δ%3= 7,56) and SED (Δ%1= 21,72; Δ%2= 26,91; Δ%3= 54,48). For the lymphocytes, the variation was ACTIV (Δ%1= 88,45; Δ%2= −48,27; Δ%3= −2,52) and SED (Δ%1= 16,81; Δ%2= −8,24; Δ%3= 7,18) and the lymphocytes subpopulations CD4+ T showed ACTIV (Δ%1= 39,91; Δ%2= −11,41; Δ%3= 23,94) and SED (Δ%1= 120,69; Δ%2= −6,47; Δ%3= 106,40); CD8+ T ACTIV (Δ%1= 38,29; Δ%2= −23,11; Δ%3= 6,33) and SED (Δ%1= 78,19; Δ%2= −15,17; Δ%3= 51,16); and CD4+ / CD8+ T ACTIV (Δ%1= 1,49; Δ%2= 16,18; Δ%3= 17,91) and SED (Δ%1= 23,08; Δ%2= 12,50; Δ%3= 38,46).
DISCUSSION
This study demonstrates the involvement of the immune system in the adaptation to BFR training and elucidates the mechanisms involved in such adaptation. In addition, the effects of this training method in chronically infected and immunosuppressed HIV patients were documented. In neither case studied, immunosuppression was observed above 30 minutes after the end of the training session, as showed in a study that demonstrated a significant increase in the count immediately post-exercise with a decrease in leucocyte count only 30 minutes after and 24 hours post-training (Souza et al., 2019).
The leukocyte modulation observed in the patients participating in this study shows evidence of an inflammatory response caused by BFR training, followed by rapid recovery, according to Souza et al. (2019). This response caused leukocyte demargination, resulting in leukocytosis (an increase in the number of circulating leukocytes). Other investigators have explained this increase by shear stress, increased circulating catecholamines, and increased cardiac output (Baganha, 2009; Walsh et al., 2011). In one study, leukocyte count was reduced 30 minutes after training, reaching a lower number than that observed in baseline or pre-exercise levels; this corroborates the findings of Dias et al. (2009) and is a likely consequence of leukocyte redistribution and apoptosis (Walsh et al., 2011).
In addition to inflammatory mechanisms, Raastad et al. (2003) observed an inversely proportional relationship between leukocyte accumulation and muscle strength after training. Due to this relationship, leukocyte modulation may be considered a valid and reliable marker of damage to skeletal muscles (Paulsen et al., 2012). Based on this information, it can be deduced that BFR training promoted tissue damage and functional adjustments of strength immediately after training in patients with HIV/AIDS.
Neutrophils are the first cells to arrive at the inflammation site and greatly contribute to leukocytosis following exercise (Peake, Nosaka, & Suzuki, 2005). Catecholamines have been indicated as one of the main causes of this neutrophilia, which results from an increase in muscle blood flow and a reduction in neutrophil adhesion due to the downregulation of adhesion molecules (Gray, Telford, Collins, & Weidemann, 1993). Based on our data, HIV-positivity and physical aptitude do not affect neutrophil demargination and seem not to affect catecholamine levels in BFR training.
Concerning monocytes, it has been reported in the literature that HIV viral replication causes an increase in oxidative stress due to chronic activation of the immune system, which consequently leads to monocyte activation (Israel & Gougerot-Pocidalo, 1997); however, at baseline, no difference was observed between healthy and HIV-positive subjects. In response to physical exercise, the number of monocytes in the blood slightly increases (monocytosis), probably due to an increase in cortisol and catecholamine levels (Walsh et al., 2011).
In our study, a greater increase in monocyte modulation occurred in the sedentary HIV-infected patient. Once the SED showed an increase in monocytes, this suggests that being physically inactive or having a higher BMI may affect the timing of monocyte responses to BFR training. These immune cells act as inflammatory signals by producing IL-1, IL-6, IL-8, IL-15, and TNF-α (Terra, Silva, Pinto, & Dutra, 2012), removing debris, and participating in tissue remodelling (Kanda et al., 2013); monocytes may take up to two hours to reach peak count (Walsh et al., 2011).
The differences in lymphocyte count observed in this study between pre and post-training and between immediately after and 30 minutes after training corroborate published data (Todo-Bom & Mota-Pinto, 2007; Kanda et al., 2013). Because lymphocytes express a high number of ß2-adrenergic receptors and these receptors tend to increase with exercise, a more significant increase might be expected in ACTIVs.
The expression of ß2-adrenergic receptors differs among lymphocyte populations: it is higher in CD8+ T lymphocytes than in CD4+ T lymphocytes (Walsh et al., 2011). For this reason, after very intense exercise, the CD4+ / CD8+ T lymphocyte ratio could be reduced; this would be associated with immunosuppression (Leandro et al., 2007) due to a higher increase in CD8+ T lymphocytes in comparison with CD4+ T lymphocytes. However, the BFR training method used in this study led to a rise in the CD4+ / CD8+ T lymphocyte ratio that maintained immunocompetence, even though this method produces results similar to high-intensity training.
Respecting the limitations of this research, that is, a small number of participants, low volume session and analysis time course up to 30 minutes after the conclusion of the training session, yet this study shows signs of safety for the application of the strength training method with BFR for people living with HIV.
CONCLUSIONS
In conclusion, BFR promoted acute inflammation after training, shown by changes in immune cell counts. These changes did not promote immunosuppression nor represent increased secretion of Cortisol and catecholamines; instead, an increase in CD4+ / CD8+ T lymphocyte ratio was observed; and HIV-infected came similar results.
This study shows similar results in the effectiveness of BFR training for booth HIV-infected patients undergoing HAART and have similar results between a sedentary and a ACTIV. However, additional studies with larger sample sizes are needed to confirm this hypothesis. In future studies, immune system cell functions and the possible effects associated with cytokines, chemokines, adipocytokines, and hormones should be analysed.