Introduction
Knowledge about COVID-19 increases every day, while the novel coronavirus spreads across the globe. Among millions of people who may be affected, patients with cardiovascular and oncologic diseases exhibit the highest risks of having worse outcomes (Liu et al., 2020; Yang et al., 2020; Zhou et al., 2020).
The American Heart Association, in conjunction with the United States´ National Institutes of Health, has just published the annual report annually on the most up-to-date statistics related to heart disease, stroke, and cardiovascular risk factors(Virani et al., 2020). This report shows that according to the 2017 National Health Interview Survey, the age-adjusted prevalence of all types of heart disease was 10.6% in the United States(Virani et al., 2020). The aging and growth of populations all over the world contribute to cardiovascular disease prevalence. In fact, almost a third of all deaths globally were due to cardiovascular disease in 2017 (Collaborators, 2018). This is not different in Brazil, where cardiovascular diseases are the major cause of death, accounting for nearly 20% of all deaths in adults(Mansur & Favarato, 2012).
Most cardiovascular diseases are chronic and need continuous care with frequent visits to medical facilities. In fact, cardiovascular ambulatory care has a pivotal role in reducing cardiovascular deaths(Tu et al., 2017). Aristizábal et al(Aristizábal et al., 2015), found a 40% reduction in emergency room visits and rehospitalizations related to new cardiovascular and coronary events in patients with a previous acute coronary event who received care under a comprehensive ambulatory care model. In Brazil, not only ambulatory services, but also the family medicine program(Silva et al., 2019) reduced hospitalizations due to cardiovascular disease(Lentsck & Mathias, 2015).
When the World Health Organization upgraded COVID-19 to pandemic status, most countries urged to promote social distancing, which means shutting down schools, prohibiting group gatherings and public events, working from home (as much as possible) and staying away from each other as much as possible. Social distancing instructions also closed many health facilities considered non-essential. Although there is no universal definition for “essential health care”(Monekosso, 1984), social distancing policies and the shortage of health staff and medical supplies resulted in closing or reducing operating hours of most public and private ambulatory general practice services.
With this scenario, we can anticipate that soon, patients with COVID-19 infection symptoms and patients with acute decompensation of cardiovascular diseases will be sharing seats in emergency waiting rooms. Thus, screening measures that can identify and isolate potential COVID-19 cases, could prevent disease transmission in health facilities.
Jiang et al. (Jiang et al., 2020) published a brief review summarizing published studies as of late February 2020 on the clinical features, symptoms, complications, and treatments of COVID-19 and found that the main clinical manifestations are fever (90% or more), cough (around 75%), and dyspnea (up to 50%). As fever is the most prevalent symptom, it seems reasonable that this vital sign should be screened as soon as patients arrive to health facilities. Most health facilities use probe electronic thermometers, which must be in contact with patient´s skin or mucosa for some minutes before temperature can be taken. After that, they should be properly disinfected before the next use. This would limit screening speed.
Objective
The aim of this paper is to discuss the potential use of infrared thermography for early detection of patients infected with COVID-19 in health facilities, proposing its use for risk reduction of cross infection.
Literature review
Infrared thermography
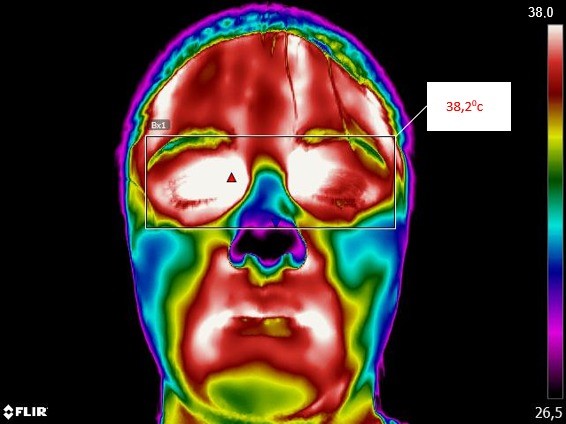
Infrared thermal imaging, or thermography, is a noncontact and noninvasive approach that has been used in medicine since the early 1960s(Ghassemi et al., 2018). Infrared thermal imaging does not require irradiation as it uses infrared radiation emitted from biological tissues to calculate temperature distributions(Usamentiaga et al., 2014). The last five decades witnessed the increase in the utility of thermal imaging cameras to correlate skin temperature and thermal physiology(Jiang et al., 2005; Merla & Romani, 2006; Ring & Ammer, 2012; Ring et al., 2010) and they are currently being used in oncologic imaging(Arora et al., 2008; Lee et al., 2010; Wishart et al., 2010), ischemic monitoring(Bagavathiappan et al., 2009; Peleki & da Silva, 2016), sports medicine(Moreira et al., 2017) and also fever screening(Childs, 2018; Dagdanpurev et al., 2018; Ghassemi et al., 2018) (figure 1).
All objects or bodies with temperature above absolute zero emit electromagnetic radiation, which is known as infrared radiation or thermal radiation(Jones, 1998). The infrared energy emitted from an object or person is directly proportional to its temperature. Infrared cameras create an image by converting radiant heat energy into a signal that can be displayed on a monitor. Therefore, temperatures are accurately measured by the infrared camera, where pixels are the data acquisition points for thermal temperature.
Infrared thermography for fever screening in individuals
Comparison of infrared temperature readings and oral temperatures has shown high sensitivity and specificity in different studies (Chan et al., 2004; Ng et al., 2004; Nguyen et al., 2010). Chamberlain et al.(Chamberlain et al., 1995) evaluated 2447 subjects aged 12 to 103 years who denied recent potentially febrile illness and ingestion of medications that affect normal body temperature. The mean ear infrared emission temperature was 36.51 ± 0.46oC. The reproducibility was better than that of electronic thermometer at the oral and axillary sites. Based on this study, the 99th percentile was 37,6oC, which the authors(Chamberlain et al., 1995) considered the appropriate cutoff for fever screening using infrared ear thermometers.
International organizations, as the International Eletrotechnical Commission(IEC/ISO, 2017) and the European Association of Thermology(Mercer & Ring, 2009) conducted clinical studies that proved the accuracy of infrared thermometers for fever screening, as far as appropriate procedures are applied. Noteworthy, there are standard and technical reports recommending best practices for thermographic fever screening(Ghassemi et al., 2018; IEC/ISO, 2017; ISO, 2009).
Since ambient temperature influences body temperature, the deployment of these systems could be more challenging in hot climates, either in tropical or subtropical regions or temperate countries during summer months. Nevertheless, Tay et al(Tay et al., 2015) found high sensitivity and specificity for fever detection using and infrared thermal detection system in a tropical healthcare setting. Also, Suzuki et al.(Suzuki et al., 2010), found that measurement of body temperature with infrared thermometer was effective for mass body temperature screening even in warm environment. Most studies analyzing feasibility of medical infrared imaging took thermal pictures in climatized rooms. Nevertheless, a practical and feasible protocol for its use in emergency rooms, where temperature is not easily controlled, is available(Coats et al., 2018).
Infrared thermography for mass detection of fever in travelers
The first studies describing infrared thermography use for mass fever screening in airports were published in 2004 (Chan et al., 2004; Ng et al., 2004). Infrared thermal cameras and noncontact infrared thermometers are the only viable temperature measurement approaches for mass screening of infectious disease pandemic(Ghassemi et al., 2018) like the current COVID-19 outbreak.
Diverse national efforts implemented the use of infrared thermography for mass detection of fever at boarders and quarantine stations. After WHO´s global alert for H1N1 pandemic in 2009 many national health agencies start to screen travelers to delay local transmission. Cowling et al (Cowling et al., 2010). reviewed these screening policies and found that they could delay local transmissions for 1-2 weeks. This period could be used to better planning and preparation for local epidemics.
Cho & Yoon(Cho & Yoon, 2014) retrieved data from arrivals’ health declaration forms and questionnaires for febrile arrivals at an international airport collected by a Korean quarantine station during 2012 and found that thermal camera temperature and tympanic (or ear) temperature was not statistically significant. Despite low fever prevalence, this manuscript suggests that self-reported questionnaires and thermal camera scanning may serve as effective tool for mass detection of fever.
During the Ebola virus disease outbreak (2014 to 2016) in Sierra Leone all people (n=166,242) passing through their International airport underwent screening with fixed infrared thermal scanners and five individuals were denied air travel from Sierra Leone(Wickramage, 2019). Since 2006, the Taiwan Notifiable Diseases Surveillance System for dengue fever has been using remote-sensing infrared thermography and quarantine stations of all harbors and international airports to detect febrile passengers(McKerr et al., 2015). This simple and robust system enabled timely and accurate reporting of dengue fever cases.
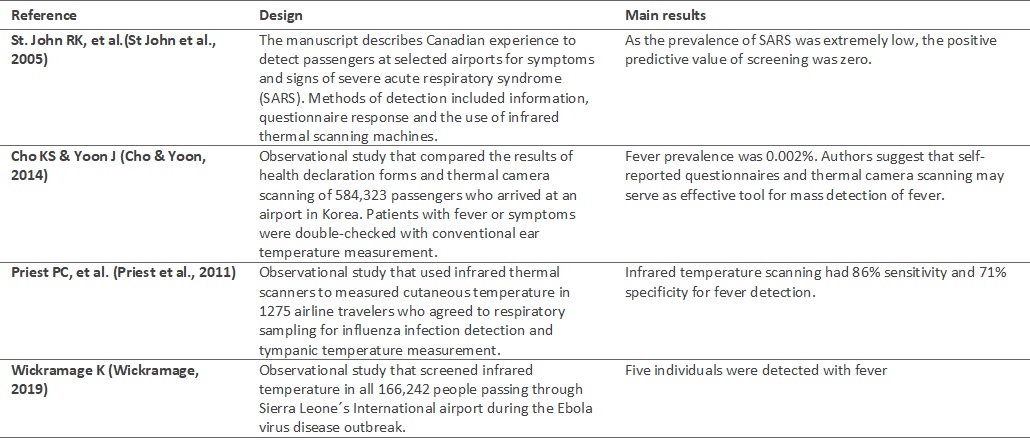
Studies that evaluated the implementation of mass fever detection in airports faced the same limitation: there were very few patients with fever (table 1) (Cho & Yoon, 2014; Priest et al., 2011; St John et al., 2005; Wickramage, 2019). Thus, it is difficult to justify the implementation of a screening system that uses new technology and personnel efforts to detect a rare condition (fever in airports).
Infrared thermography for detection of fever in health facilities
As prevalence of fever is expected to be higher in medical facilities, mass screening of individuals entering hospitals should be detect more people with fever in hospitals than in airports. A prospective observational study(Holm et al., 2018) included 198 medical patients admitted to the Emergency Department. Researchers took standardized thermal picture and temperatures of the inner canthus (central temperature) and three peripheral temperatures (earlobe, nose tip, and tip of the third finger). Gradients between central and peripheral temperatures showed a significant association with 30-day mortality, suggesting prognostic value.
During the severe acute respiratory syndrome (SARS) epidemics in 2003, Taiwan implemented a protocol where all patients and visitors should be screened for fever at the entrance of every hospital building, aiming to reduce the risk of nosocomial cross infections. The infrared thermal imaging system screened 72,327 outpatients and visitors at the Taipei Medical University-Wan Fang Hospital and detected 305 febrile patients(Chiu et al., 2005), three of them with SARS.
Reducing cross-infection of patients with cardiovascular disease in medical facilities
During pandemics medical services are overwhelmed and patient triage is of paramount importance. Unfortunately, healthcare resources are limited, and patients are facing long waiting times in medical facilities. It is important to identify and prioritize patients who requires urgent attention and intervention (Tam et al., 2018). In pandemic times, proper and fast patient triage can also guide right-hospital allocation accordingly to possible infective status. If fast triages strategies are not implemented, patients with cardiac decompensation and with COVID-19 infection will be sharing the same waiting rooms, increasing the risk of cross-infection. Noteworthy, symptoms-focused triage evaluation may not be effective in identifying infected patients, as patients with cardiovascular decompensation and the ones infected with COVID-19 share symptoms (breathlessness, fatigue and chest pain).
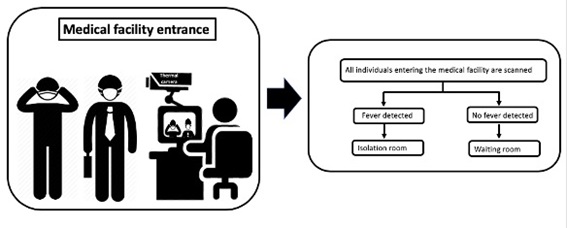
As temperature readings obtained by infrared thermal imaging system could be used to screen patients and visitors in medical facilities(Ataş Berksoy et al., 2018; Chiang et al., 2008) we propose a flowchart (Figure 2) to guide the thermal screening of everyone entering medical facilities. Each person with high temperature should be rapidly isolated and evaluated. This relatively simple flowchart could potentially reduce cross-infection risk at waiting rooms.
Limitations of infrared thermography implementation in medical facilities
Despite the promising results of infrared thermography use in emergency rooms, there are some limitations that must be considered. Asymptomatic patients may transmit the virus (Wei et al., 2020). While thermography is useful to identify patients with elevated central temperature, it would not be able to identify those asymptomatic patients with temperature values within the normal range. Nevertheless, asymptomatic patients would seldom, if ever, seek medical assistance during pandemics. Noteworthy, taking of an antifebrile drug results in body temperature reduction, which can affect thermography efficacy (Chiang et al., 2008; Nishiura & Kamiya, 2011).
Currently available infrared cameras show high sensitivity, excellent time resolution and should be calibrated according to heat emissivity, room temperature, humidity, and distance to the object of interest. Most published studies lack complete and detailed descriptions of how the camera and/or the software were calibrated and what settings have been used, limiting their reproducibility (KJ et al., 2020; Shterenshis, 2017).
Finally, normative range of surface skin temperature is still not established(Shterenshis, 2017) and accuracy of fever detection by infrared thermography is determined by the selected fever temperature cutoff. Noteworthy, the use of different cutoff values would impact in method´s sensitivity and specificity. In fact, maximizing accuracy by choosing highest specificity may not be desirable in a real-world pandemic setting, where secondary evaluation is available. By the other hand, setting thermo scanners to high sensitivity would increase the demand to secondary evaluation.
Conclusion
Patients with comorbidities and chronic disease, especially the ones with cardiologic or oncologic diseases, are at increased risk of bad prognosis when infected by COVID-19(Liu et al., 2020; Yang et al., 2020; Zhou et al., 2020). Despite social isolation policies, these patients must keep continuous healthcare to reduce disease decompensation, emergency room visits and rehospitalizations.
Limiting contact between infected and non-infected patients is pivotal to reduce nosocomial cross infections during the COVID-19 pandemic. Previous experiences show that infrared thermography is a radiation-free, relatively inexpensive, noncontact, and noninvasive technology that could be used for mass-screening of patients and visitors in health care facilities. Implementation of thermography use in healthcare facilities entrance can detect and rapidly isolate people with fever, providing them appropriate care and reducing nosocomial cross-infection.