Introduction
One of the major advantages that point of care ultrasound (POCUS) gives clinicians is the ability to assess physiology at the bedside, particularly with Doppler technology making flow assessment possible. The practice of medicine is also filled with dogmatic practices usually based on valid general concepts not applicable to all patients but nonetheless often used as gold standards. One of these prevalent beliefs is that fluids are universally beneficial - or at least not harmful - to renal function, while in fact, there is no actual literature supporting this concept, and in fact a good amount of literature showing how central venous pressures (CVP) above the range of 6-8 are associated with worse renal outcomes.1 This common belief results in inappropriate fluid administration, or else withholding/decreasing diuretic therapy when it isn’t the correct clinical management.
Recently, solid organ Doppler has expanded the assess-ment of venous congestion and brought measurable variables to the bedside and has been synthesized into a splanchnic congestions score known as the venous excess ultrasound score (VExUS).2 Here, we present a case where using the VExUS score was able to assist the clinicians in guiding the management of a complicated case.
Case Report
A 32-year-old male patient, smoker, with no history of diabetes or hypertension, was admitted for dyspnea at rest, palpitations and anasarca with a presumptive diagnosis of acute decompensated heart failure (ADHF). The patient had a history of pericardiectomy for constrictive pericarditis, diagnosed based on cardiac magnetic resonance imaging (MRI) with gradual deterioration in the month prior to admission. Operative pathology had revealed marked fibrosis with mild inflammatory cell infiltrate and foci of dystrophic calcification, without evidence of specific granuloma or malignancy. On examination, the patient was fully conscious, temperature was 36.8ºC, blood pressure (BP) 125/70 mmHg, heart rate (HR) 114 bpm, respiratory rate (RR) 38 per minute, neck veins were distended, oxygen saturation (SpO2): 81% on room air. Pansystolic murmur heard over the tricuspid area, bilateral diminished air entry more on the right side of the chest, increased abdominal girth with abdominal wall edema and lower limbs showed bilateral massive non-pitting edema reaching the abdomen. Central venous pressure (CVP) was 26, PH: 7.38, PCO2: 27 mmHg, PO2: 58 mmHg, HCO3: 17 mmol/L, SO2: 81%, hemoglobin (Hb): 8.6 g/dL, total leukocytic count (TLC): 13 000 /uL, platelets count (PLT): 207 000 /uL, INR: 1.3, urea: 131 mg/dL, creatinine: 3.1 mg/dL, estimated glomerular filtration rate (eGFR): 46 mL/min, sodium (Na): 131 mmol/L, potassium (K): 4.8 mmol/L, albumin: 3.1 g/dL, alanine aminotransferase (ALT): 541 U/L, aspartate aminotransferase (AST): 463 U/L, total bilirubin: 4.12 mg/dL, direct bilirubin: 2.7 mg/dL, C reactive protein (CRP): 76 mg/L, transferrin saturation: 5.8%. Urine analysis revealed: pus cells: 70, RBCs: 20-30, bacteria: ++, urine culture revealed klebsiella species. A computerized tomography (CT) scan of chest was done and revealed moderate pericardial effusion showing pericardial calcifications, features of constrictive pericarditis. Moderate right and encysted pleural effusions. No signs of consolidative pneumonia. Transthoracic echocardiography (TTE) using the commercially available machine (Philips Affiniti) revealed thickened pericardium, left ventricle ejection fraction (LVEF): 61%, left ventricular end diastolic diameter (LVEDD): 3.9 cm, left ventricular end diastolic diameter (LVESD): 2.5 cm, pulmonary artery systolic pressure (PASP): 60 mmHg, tricuspid annular plane systolic excursion (TAPSE): 1.42 cm, moderate tricuspid regurgitation (TR) and distended right ventricle with exaggerated ventricular interdependence (Video 1). Pulsed wave Doppler recording of tricuspid and mitral inflow velocities showing marked respirophasic variation (Fig. 1). Tissue Doppler imaging (TDI) of the lateral and medial mitral annulus showing higher E' velocity at the medial side (annulus reversus) contrary to normal (Fig. 2).
Video 1: Transthoracic echocardiography. 2D transthoracic echocardiography, apical four chamber view showing septal bounce (oscillatory diastolic beat-to- beat movement), distended right ventricle with exaggerated ventricular interdependence

Figure 1: Pulsed wave Doppler recording of tricuspid and mitral inflow. Pulsed wave Doppler recording of tricuspid (A) and mitral (B) inflow velocities showing marked respirophasic variation, A: Tricuspid inflow velocities increase with inspiration and decrease with expiration (Ee and Ae). B: Mitral inflow velocities increase during expiration (Ee and Ae) and decrease with inspiration (Ei and Ai).
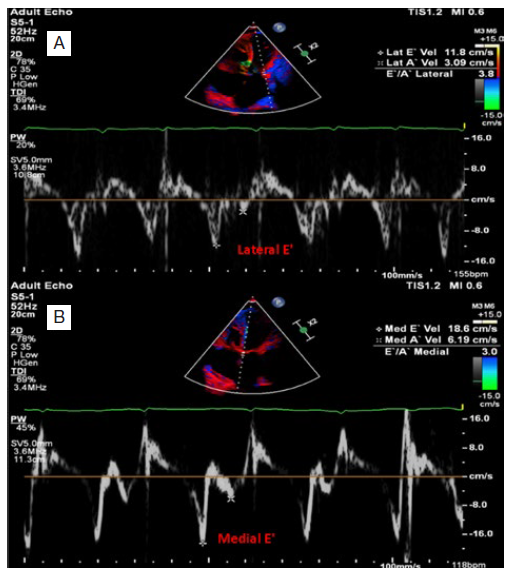
Figure 2: Tissue Doppler imaging (TDI). Tissue Doppler imaging (TDI) of the lateral (A) and medial (B) mitral annulus showing higher E' velocity at the medial side (annulus reversus) contrary to normal.
A continuous intravenous (IV) infusion of furosemide was started, besides antibiotics and oxygen. Pleural effusion was drained, yielding 3 Lof transudate. After achieving a 26 L negative cumulative fluid balance over 18 days, the patient clinically improved significantly, and BP 110/70 mmHg, RR 27, SPO2 92% on 2 L nasal prongs. Serum creatinine decreased to 1.8 mg/dL but fluctuated to 2 mg/dL. The diuretic dose was decreased and changed to intermittent dosing (average 80 mg/d) targeting a less intense negative balance for fear of further renal deterioration. But this did not achieve any further improvement of serum creatinine and the patient was still oxygendependent. So, assessment with venous excess ultrasound (VExUS) was done using the cardiac phased array probe.
The IVC was plethoric and poorly collapsible (maximum diameter 2.3 cm & minimum diameter 2.2 cm), hepatic vein Doppler showing diastolic dominant antegrade component (S<D) (mildly abnormal H.V) (see Fig. 3A). Fig. 4 shows the individual waves of hepatic vein trace, portal vein Doppler showing severe pulsatility, pulsatility index 68% (severely abnormal) (see Fig. 3B), renal vein Doppler showing a monophasic pattern (severely abnormal) (see Fig. 3C), these findings correspond to VExUS grade 3. So, intravenous furosemide infusion was restarted, targeting a more aggressive negative balance (3 L per 24 hours), and after 72 hours and achieving a negative 8.5 L fluid balance, body weight decreased to 77 kg with BP 110/60 mmHg, HR 98, RR: 23, SPO2: 93 room air.
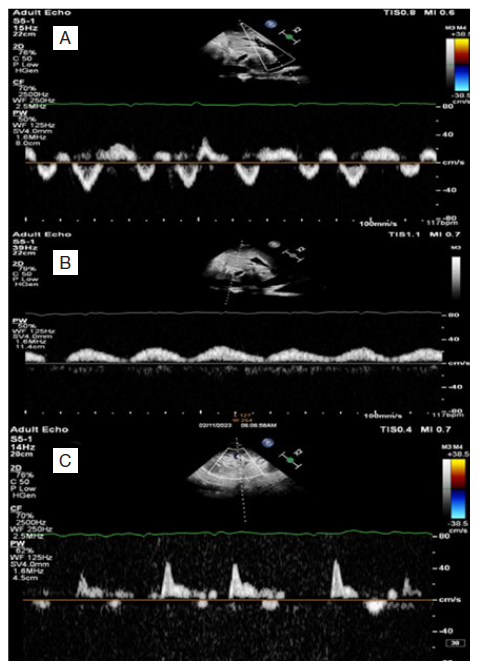
Figure 3: VExUS grading system, first study. Pulsed wave Doppler of hepatic veins showing diastolic dominant antegrade component (S<D) with DR wave which is characteristically seen in constrictive pericarditis (A), pulsed wave Doppler of portal vein, severely pulsatile (pulsatility index 68%) (B), pulsed wave Doppler of renal inter-lobar vessels, showing a monophasic pattern (D wave) (C).
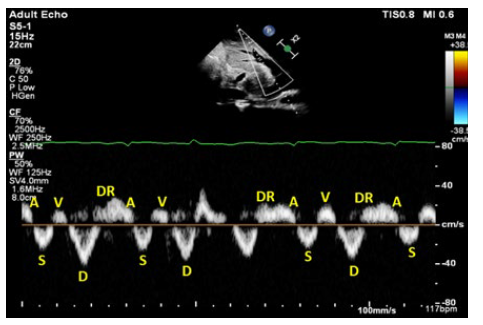
Figure 4: Individual waves of hepatic vein trace, first study A, S, V, D and DR represent individual waves in the hepatic vein trace, which showing diastolic dominant antegrade component (S<D) with DR wave which is characteristically seen in constrictive pericarditis.
The IVC is still plethoric and poorly collapsible (maximum diameter 2.29 cm & minimum diameter 2.24 cm), hepatic vein Doppler showing diastolic dominant antegrade component (S<D) (mildly abnormal H.V) (see Fig. 5A), portal vein Doppler showing diminishing pulsatility, pulsatility index 46% (mildly abnormal) (see Fig. 5B), renal vein Doppler showing a biphasic pattern (mildly abnormal) (see Fig. 5C), these findings correspond to VExUS grade 1. Follow-up laboratory assessment revealed, Hb: 9.5 g/dL, TLC: 7.6 K /uL, platelets: 289 K /uL, INR: 0.9, urea: 94 mg/dL, creatinine: 1.4 mg/dL (eGFR : 80 mL/min), Na: 134 mmol/L, K: 4.1 mmol/L, albumin: 3 g/dl, ALT: 21 U/L, AST: 18 U/L, total bilirubin: 0.8 mg/dL, direct bilirubin: 0.52 mg/d. The patient improved clinically and laboratory, he was transferred to the ward and after 2 days, he was discharged with serum creatinine 1.2 mg/dL on oral spironolactone with good urine output, and after one month of discharge, he is in a good condition with no need for rehospitalization waiting for redo pericardiectomy.
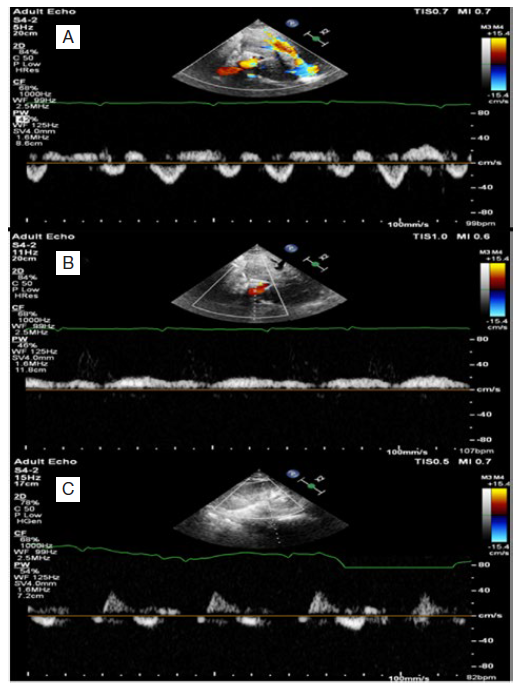
Figure 5: VExUS grading system, follow-up study. Follow-up pulsed wave Doppler of hepatic vein showing diastolic dominant antegrade component (S<D) (A), pulsed wave Doppler of portal vein showing diminishing pulsatility index (pulsatility index 46%) (B), pulsed wave Doppler of renal interlobar vessels (biphasic pattern) (C).
Discussion
This case illustrates well the inherent limitations of the traditional examination and how POCUS, by being integrated into the physical examination, can help overcome these. Indeed, it is easy to understand how clinicians, faced with a net negative balance of 26 L and some renal dysfunction, may be wary of continuing aggressive diuresis. However, armed with the bedside understanding of the patient’s pathophysiology - in this case VExUS 3 score with severe intrarenal venous Doppler abnormality of diastolic-only flow - the treatment plan becomes clear and can be implemented with much less hesitation or concern.
Recently, a study was published showing how marked congestion by VExUS score was associated with AKI in patients with acute coronary syndromes.3 It is important to note that while venous congestion is caused by a number of different pathologies, it is not cardio-centric, but organ-centric. Congestion is felt by the organs, irrespective of the pathology that causes it. Certainly the outcomes may vary as patients’ baseline organ function do as well, and there is an element of chronic adaptation that seems to take place -perhaps related to improved lymphatics or perhaps cellular adaptation. Hence the importance of measuring the venous afterload that the organs are faced with. This has also been done by using the renal venous stasis index, which is very similar in nature to the VExUS score but is limited to the renal vasculature.4
Conclusion
While further studies are clearly needed to establish decongestion targets potentially based on VExUS scoring, it is clear and consistent from the existing literature that elevated levels correlate with organ dysfunction, notably acute kidney injury. Hence the finding of severe congestion by VExUS should suggest to the clinician that a component of the renal dysfunction may be congestive in nature and prompt a trial of decongestion. Naturally, this needs to be done with intense follow-up of clinical, ultrasound and laboratory evolution.
We look forward to randomized trials establishing venous assessment as a foundation of management of congestive states.















