Introduction
Drug induced liver injury (DILI) is a well-recognized condition with a wide spectrum of symptoms that can mimic acute and chronic liver diseases. Being able to identify the substance implicated is crucial, since the first-line treatment consists of withdrawing the toxin. There are over 1000 drugs and herbal products described as potential inducers of DILI.1 When there is a temporal relationship between the onset of a drug administration and the appearance of liver changes, the diagnosis becomes more evident. However, this diagnostic hypothesis is not always obvious, especially when dealing with pharmacological substances without a previous and clear knowledge of causing liver injury. Amongst those drugs is one of the most recent group of oral antidiabetic drugs.
Sodium-glucose cotransporter 2 inhibitors (SGLT-2i) prevent glucose reabsorption in proximal renal tubule resulting in glucose renal excretion and subsequent reduction in serum levels. Four specific drugs (canagliflozin, dapagliflozin, empagliflozin and ertugliflozin) were introduced in clinical practice. Several adverse effects have been described, but none of the four agents was associated with increased serum aminotransferase or alkaline phosphatase in clinical trials, and since licensure, there have been only very rare and not completely conclusive reports2 of clinically apparent induced liver injury.
Thereby, we present a rare clinical case of a diabetic fe-male patient that develops DILI after initiating empagliflozin 1 month earlier.
Case Report
A 70-year-old Caucasian woman presented with a 3-day continuous diffuse abdominal pain associated with nausea and diarrhoea (without blood, mucus or pus). The patient denied fever or any other symptoms.
She had a long-time history of poorly controlled type 2 diabetes with target organ damage, especially diabetic nephropathy, and severe ischemic heart disease with the need for primary percutaneous angioplasty one year before, due to myocardial infarction (being medicated with acetylsalicylic acid 100 mg/day and ticagrelor 180 mg/day since then). It is noteworthy the introduction of empagliflozin in the last month, aiming to achieve a better disease control, despite glargine insulin therapy. Other relevant past medical history includes obesity, arterial hypertension (well managed with valsartan 80 mg/day, amlodipine 5 mg/day, carvedilol 25 mg/day and furosemide 40 mg/day), dyslipidaemia (under atorvastatin 20 mg/day) and anxiety/depression disorder (medicated with trazodone 100 mg/day, amitriptyline 25 mg/day and pregabalin 150 mg/day). There was no alcohol, drug or any natural products consumption. She denied animal contact and was a pastry chef for a living.
On physical examination she revealed diffuse pain, more intense on the upper right quadrant, without peritoneal irritation signs or Murphy’s sign. The remaining of her physical examination was unremarkable.
Laboratory investigation revealed an elevation of both cytolytic and cholestatic parameters (aminotransferases greater than ~4 times the normal upper limit; alkaline phos-phatase and gamma-glutamyl transferase increased ~6 and 18 times than the normal upper limit, respectively) despite normal albumin, bilirubin, and coagulation function tests (Table 1). Abdominal and kidney ultrasounds were performed and reported normal, except for vesicular microlithiasis. Thus, she was hospitalized for further investigation, after empagliflozin withdrawal.
Table 1: Abnormal blood tests results
| Blood test | Result | Normal range |
|---|---|---|
| Creatinine | 1.6 mg/dL | 0.5-1 mg/dL |
| Lactate dehydrogenase | 445 U/L | < 247 U/L |
| Aspartate aminotransferase | 123 U/L | < 31 U/L |
| Alanine aminotransferase | 248 U/L | < 34 U/L |
| Gamma-glutamyl transferase | 553 U/L | < 38 U/L |
| Alkaline phosphatase | 725 U/L | 30-120 U/L |
| C-reactive protein | 4.3 mg/dL | < 0.5 mg/dL |
Blood and urine cultures and serologies for hepatotrophic pathogens (Table 2) were performed and reported negative. Hepatic autoimmune diseases were excluded, and no other immunologic alterations were identified. There was no evidence of copper or iron excess. Since vesicular microlithiasis was described, despite the absence of ultrasound signs of cholecystitis or choledocholithiasis, a magnetic resonance cholangiopan-creatography (MRCP) was carried out. However, no pathological lithiasic condition or biliary tract dilatation was evident.
Table 2: Blood serologies (hepatotrofic virus and bacteria)
| Microorganism | Result | Interpretation |
|---|---|---|
| Cytomegalovirus | Positive IgG, negative IgM | Previous infection |
| Herpes simplex virus 1 | Positive IgG, negative IgM | Previous infection |
| Herpes simplex virus 2 | Negative IgG and IgM | No active infection |
| Epstein-Barr virus | Negative IgG, IgM and EBNA | No active infection |
| Hepatitis A virus | Negative IgG and IgM | No active infection |
| Hepatitis B virus | Negative HBsAg, HBsAb and HBcAb | No active infection |
| Hepatitis C virus | Negative IgG and IgM | No active infection |
| Hepatitis E virus | Negative IgG and IgM | No active infection |
| Human immunodeficiency virus | Negative Ag/ Ab | No active infection |
| Treponema pallidum | Negative IgG and IgM | No active infection |
| Rickettsia conorii | Negative IgG and IgM | No active infection |
| Coxiella burnetii | Negative IgG and IgM | No active infection |
IgG - immunoglobulin G; IgM - immunoglobulin M; EBNA - Epstein-Barr nuclear antigen; HBsAg - hepatitis B surface antigen; HBsAb - hepatitis B surface anti-body; HBcAb - hepatitis B core antibody; Ag- antigen; Ab - antibody.
According to the findings, the hypothesis of liver toxicity secondary to empagliflozin became the most likely. Although there was a progressive normalization of liver enzymes after empagliflozin suspension, a liver biopsy was needed to enhance the hypothesis. Non necrotizing lobular microgranulomas (Fig.s 1 e 2) were found and there was no inflammatory infiltrate, loss of biliary ducts or visualization of microorganisms, suggesting the diagnosis of liver toxicity.
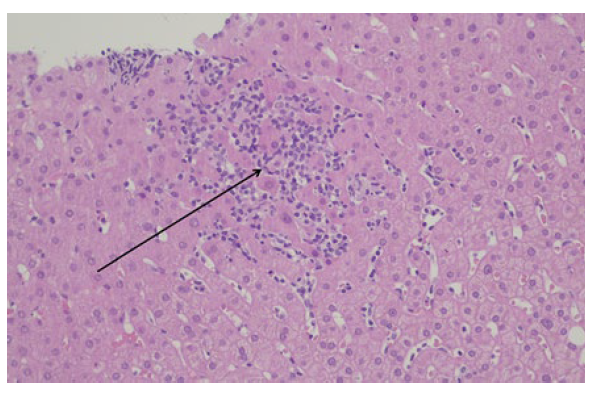
Figure 1: Liver parenchyma with microgranulomas (arrow) (hematoxylineosin staining, 200x magnification).
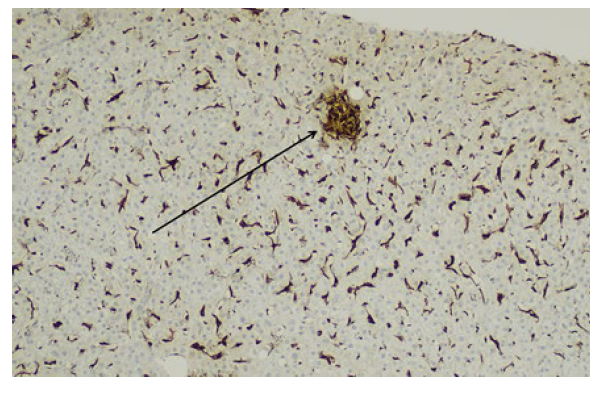
Figure 2: A microgranuloma is highlighted by the CD68 stain (arrow), which also exhibits Kupffer cell hyperplasia (100x magnification).
The acute toxic hepatitis was attributed to empagliflozin, introduced 1 month earlier. The remaining drugs that could induce liver toxicity were not discontinued, given their chronicity over more than 1 year.
She had a favourable evolution and was discharged after 2 weeks of hospitalization with a reinforced dietetic, exercise and insulin therapy plan. At 1-month follow-up she was asymptomatic, without analytical changes (Fig. 3).
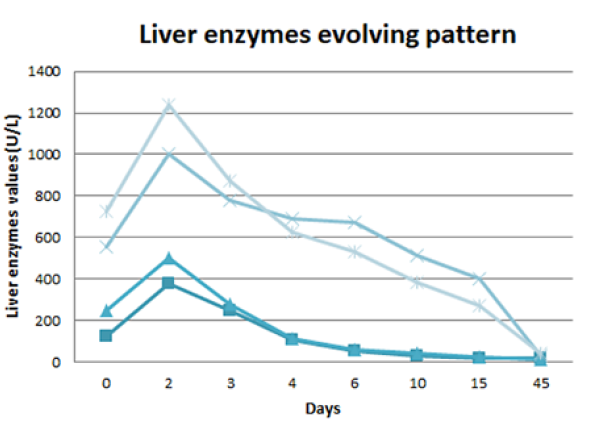
AST - aspartate aminotransferase. ALT - alanine aminotransferase. GGT - gamma - glutamyl transferase. FA - alkaline phosphatase
Figure 3: Liver enzymes evolving pattern: progressive normal-ization of liver enzymes during the 15 days of hospitalization, with a slower lowering of cholestatic enzymes. One month after discharge (45 days after being admitted to the emergency department) there was a complete normalization of the hepatic alterations.
Discussion
DILI accounts for <1% of cases of acute liver injury but it is the most common cause for acute liver failure in the USA and Europe. This condition is one of the main reasons for drug withdrawal. In contrast to intrinsic DILI, the onset of idiosyncratic DILI, which is less common but nonetheless responsible for about 10%-15% of acute liver failures in the USA, is almost impossible to predict, being characterized by a variable latency to onset (weeks to months) and a lack of clear dose dependency.3
Once DILI is induced by many drugs, herbs, and dietary supplements it can represent a diagnostic challenge, particularly in polymedicated patients. The development of nonspecific symptoms after introducing a new drug may indicate drug toxicity and an evaluation for DILI should be considered. A high clinical suspicion after a thorough history is crucial for the diagnosis. Blood tests to exclude other causes of liver injury, including viral and autoimmune diseases, are also necessary. It is known that ageing influences DILI phenotypes, making cholestatic damage more likely in ≥60-year-old patients, regardless of the used drug. Therefore, MRCP is indicated to rule out biliary obstruction if cholestasis is present.4
Liver biopsy may suggest the diagnosis of DILI through the histological pattern of injury and correlation with clinical history, laboratory, and imaging data. When alternative causes for liver injury have been excluded and the patient has been exposed to a drug known to induce hepatic injury, DILI can be assumed as final diagnosis and no liver biopsy is necessary. In fact, liver biopsy is not routinely used in the evaluation of suspected DILI, except when the diagnosis or the injury severity remains uncertain. After excluding the main causes for necrotizing microgranulomas (such as sarcoidosis and tuberculosis), the probability of them being induced by drugs is high.5 Despite this, it is noteworthy that complete confirmation of DILI may require following the pa-tient long past the point of liver biopsy and in our patient, we verified a complete normalization of liver changes after empagliflozin withdrawal.6
Empagliflozin is a SGLT-2i that can be safely used in mono or combined therapy, due to its insulin independent action mechanism. Empagliflozin was approved in 2014 for management of hyperglycaemia in type 2 diabetes. In 2017, the indications to its use were extended to decreasing the risk of cardiovascular death in adults with type 2 diabetes and associated cardiovascular disease.
In clinical trials phase III empagliflozin was well tolerated and most adverse effects were mild to moderate, leading to discontinuation of the drug in a similar proportion to the placebo group. The only official contraindication to the use of empagliflozin is hypersensitivity to the correspondent substance.7
DILI by any of the SGLT-2i is quite rare and this may be related to their minimal hepatic metabolism which is largely via uridine diphosphate glucuronylsyltransferase. A prospective study carried out between 2004 and 2013 identified 899 cases of DILI, but none attributable to SGLT-2i.8 Indeed, a systematic review of 18 controlled trials of type 2 diabetic patients treated with SGLT-2i found out that this pharmacological group not only was not associated to hepatotoxicity, but in fact reduced ALT levels compared to placebo (by an average of 2.8U/L).9
In multiple randomized controlled trials, empagliflozin was not associated with liver enzymes elevation during the-rapy.10 By the contrary, there are trials that can identify a reduction in cytolysis and/or cholestasis parameters for all the SGLT-2i, probably as a result of concurrent improvement in fatty liver disease due to a better glycaemic control, weight loss or both.11During prelicensure studies, no clinically apparent acute liver injury was convincingly linked to empagliflozin12 and hepatic enzymes elevation accompanied by jaundice occurred equally in dapagliflozin13 and placebo groups. Since SGLT-2i approval and more widespread use, there have been only 2 cases of DILI reported:
1) A young female patient with non-alcoholic fatty liver disease and cirrhosis developed decompensation with jaundice, ascites and encephalopathy 10 weeks after starting dapagliflozin, having somewhat improved after stopping therapy, but ultimately required liver transplantation several months later14;
2) An elderly woman with non-alcoholic fatty liver disea-se developed fever, jaundice and liver enzymes elevation 6 weeks after starting empagliflozin, which resolved within a week after withdrawal and remained normal thereafter.15
It is noteworthy to point out that both of the cases oc-curred in patients with previous liver conditions, just like it happened with our patient, that also had hepatic steatosis.
Despite being an overall safe pharmacological group, with few known adverse effects, SGLT-2i may develop he-patotoxicity, as described previously in the literature. The fact that DILI is so rare with these drugs makes this case even more interesting, as it alerts the clinicians to an early diagnosis and appropriate treatment, with immediate sus-pension of the drug.















