Atrial fibrillation (AF) is a common form of arrhythmia and is one of the leading causes of stroke, heart failure, cardiovascular morbidity, and sudden death worldwide. The estimated prevalence of AF was between 2% and 4% worl-dwide.1 In Portugal, the prevalence of this condition among patients aged 40 or older was 2.5%, increasing to 9.0% in individuals aged 65 or older.1-5 The number of patients with AF has been rising and it is estimated that, in 2030, 14 to 17 millions of patients globally will have AF, with 120 000-215 000 being newly diagnosed every year.6,7
AF is frequently associated with structural heart disease and other comorbidities (e.g. arterial hypertension, hyperlipidaemia, heart failure, and diabetes). AF has a significant morbidity and is independently associated with increased risk of all-cause mortality as a result of hemodynamic abnormalities, thromboembolic events (about 20%-30% of ischemic strokes are due to AF), and frequent hospitalizations.6,7AF, even if symptomatic or asymptomatic and regardless of its pattern, is associated with a significantly risk of ischemic stroke (the risk of stroke is 5 times greater in non-valvular AF and 20 times greater in AF associated with mitral stenosis).6,7
Oral anticoagulation (OAC) with vitamin K antagonists (VKAs) or non-vitamin K antagonist oral anticoagulants (NOACs) decreases the risk of stroke in patients with AF and conse-quently their morbidity and mortality.6-8Over the last years, the prescription of NOACs has increased over VKAs and, within the NOACs, rivaroxaban is the most prescribed drug.9,10
A systematic review with meta-analysis of observational studies of Portuguese patients reported a prevalence of anti-coagulation in AF patients eligible for OAC of 40%.11 Two United Kingdom (UK) studies undertaken in general practice reported that 50.7%-54.7% of AF patients were on OAC.12,13Most lite-rature, however, seems to focus on the presence or omission of anticoagulation in patients with AF, but less is known about the quality of prescribing in patients already orally anticoagulated. Patients may be on the wrong OAC or may be on the right OAC but with incorrect doses that may lead to infra or supratherapeutic doses, with an increased risk of thrombotic or haemorrhagic events, respectively. Therefore, our aims were to evaluate if patients with AF were anticoagulated and to evaluate the quality of prescribing in patients already on OACs.
Material and MethodsSTUDY DESIGN AND SETTINGThis study follows the STROBE guidelines for observational studies.14 A cross-sectional study, using retrospective data, was undertaken for a four-month period (January to April 2019). The study was conducted in the Internal Medicine Department of a hospital from Alentejo. The hospital covers a resident population of about 33 677 inhabitants, with an area of influence of 8.542.7 km2, which corresponds to approximately 9.3% of the national territory.
POPULATION AND SAMPLEThe population of interest was defined as the resident population covered by this hospital, which was 33 677 inhabitants (Pordata, 2018). Patients were included in the study if they cumulatively:
1) were aged ≥ 18;
2) had previous history of non-valvular AF;
3) presented a CHA2DS2-VASc ≥ 2 points if they were male r ≥ 3 if they were female1;
4) were admitted to the Internal Medicine Department. Patients were excluded if they presented mechanical heart valve, moderate-severe mitral valve stenosis, or any contraindication for OAC use
Sample size estimation was performed using Epi Info® (v.6.0), considering a total population of 33 677, a prevalence of patients with AF using OACs of 40%,11 a beta error of 3%, and a 95% confidence interval. Therefore, our sample should consist of 1026 patients with AF.
OUTCOME DEFINITIONS AND MEASURESThe first endpoint accessed in this study was the number of patients that were using OACs, knowing that all the sample was eligible for oral anticoagulation. The second endpoint was the number of patients that were correctly anticoagulated, i.e., those that were prescribed with OACs, had the drug dose adjusted for clinical and laboratory characteristics. In the group of patients using NOACs, they were considered correctly anticoagulated if they had the drug adjusted (in terms of dose and frequency) for their age, renal function, and weight. In the group of patients using VKAs, they were considered anticoa-gulated if their last value of INR was between 2.0 and 3.0.
DATA EXTRACTIONData was extracted from medical records, which included sociodemographic (sex and age), clinical data (comorbidities, previous history of cerebrovascular events - ischemic stroke and transient ischemic attack), laboratory values (INR values and renal function), and pharmacotherapeutic profile (number of medications, pharmacotherapeutic group of OAC, active ingredient used, and dose). Renal function was evaluated using serum creatinine (mg/dL) and the creatinine clearance (mL/min; this parameter was estimated using the Cockcroft-Gault Equation). CHA2DS2VASc scores were estimated considering the 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation.1
ETHICS AND CONFIDENTIALITYThis study was approved by the Ethics Committee of the hospital where data was collected (document EDOC/2020/18845).
DATA ANALYSISData analysis was performed using uni-and bivariate statistics (IBM SPSS v.20.0). Descriptive statistics were used, where numerical variables were expressed using central tendency and dispersion measures (mean and standard de-viation) and categorical variables as absolute and relative frequencies. Variables were tested for normal distribution using the Kolmogorov-Smirnoff test. Bivariate analysis was performed using Student’s T-test for continuous variables and the chi-square test for categorical data, considering a 95% confidence interval. The goal of this analysis was to evaluate potential differences between the OAC and No-OAC groups regarding age, CHA2DS2VASc scores, renal function, and number of medications used per patient.
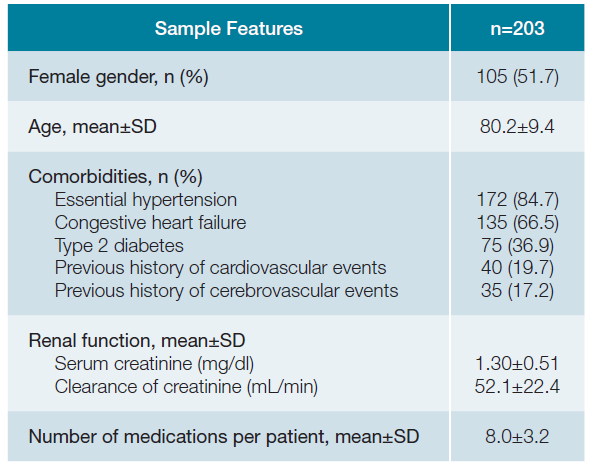
Results1. SOCIODEMOGRAPHIC AND CLINICAL FEATURESA total of 203 patients were included, where 51.7% (n = 105) were female with a mean age of 80.2 ± 9.4 years old. Most of the patients presented essential hypertension (n = 172; 84.7%) and congestive heart failure (n = 135; 66.5%), followed by type 2 diabetes (n = 75; 36.9%) and previous his-tory of cardiovascular (n = 40; 19.7%) and cerebrovascular (n = 35; 17.2%) events. Patients presented a mean serum creati-nine of 1.30 ± 0.51 mg/dL and a mean clearance of creatinine of 52.1 ± 22.4 mL/min. Patients were using a mean number of 8.0 ± 3.2 medications per patient. Detailed results are available in Table 1.
2. ANTICOAGULATION ASSESSMENT AND TREATMENT CHARACTERIZATION
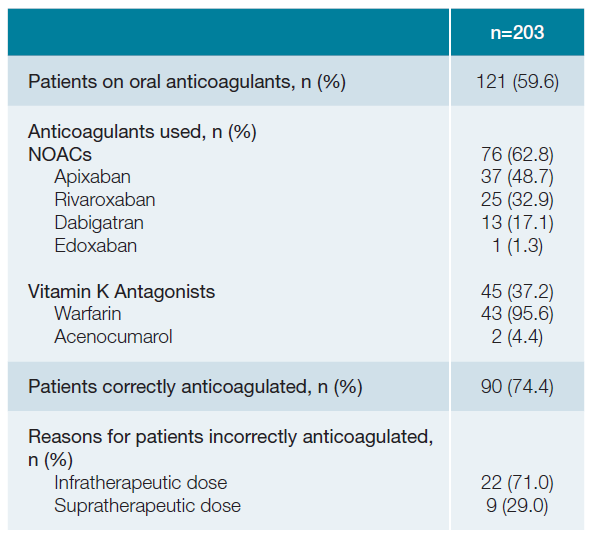
round 40% (n = 82) of patients were not on OAC. Fifty-seven percent (n = 20) of patients that had previous history of cerebrovascular events were not on OACs. About 63% (n = 76) of patients were on NOACs, where apixaban (48.7%; n = 37) and rivaroxaban (32.9%; n = 25) were the most commonly used. Thirty-seven percent (n = 45) were on VKAs, where warfarin (95.6%; n = 43) was the most commonly used drug (Table 2).
A quarter of the sample (n = 31) were incorrectly anticoa-gulated. Infratherapeutic dosing (71%; n = 22) was the main cause for patients to be considered incorrectly anticoagulated. Most patients (67.7%; n = 21) incorrectly anticoagulated were aged 75 or older.
When comparing age, CHA2DS2-VASc, renal function, and number of medications used per patient between OAC groups, only age and number of medications were statistically different (p = 0.001 and p = 0.027, respectively). Results are described in Table 3.
Table 3: Differences between oral anticoagulation groups regarding age, CHA2DS2-VASc, renal function and number of medications
| OAC (n=121) | No-OAC (n=82) | p | |
| Age, mean±SD | 78.4±9.7 | 82.8±8.4 | 0.001 |
| CHA2DS2-VASc, mean±SD | 4.6±1.4 | 4.7±1.5 | 0.947 |
| Renal function*, mean±SD | 51.3±21.8 | 53.2±23.4 | 0.561 |
| Number of medications used per patient, mean±SD | 8.5±3.3 | 7.4±3.2 | 0.027 |
*For renal function analysis was used the clearance of creatinine (ml/min)
No-OAC - not oral anticoagulation; OAC - oral anticoagulation; SD - standard deviation
Our study showed a prevalence of use of OACs by patients with AF of 59.6%, similar to what has been reported both in the UK and in the United States (US) and in a meta-analysis combining 7 studies.11,13,15Other studies, however, have showed much higher proportions of AF patients not anticoagulated, reaching 80%, despite not having any contraindication.13,15,16
In our study, about 57.1% of patients were not anticoagulated and had previous history of cerebrovascular events, a higher value than reported elsewhere.17The occurrence of ischemic stroke and transient ischemic attack in AF patients on OAC could be explained by the use of infratherapeutic doses, poor treatment adherence or another etiology other than cardioembolic.18
Our data suggests that advanced age may partly be accountable for the prescriber’s decision not to anticoagulate. Old age is associated with an increased prevalence of AF and an increased stroke risk from AF which reaches an annual risk up to 23.5% in AF patients aged 80 to 90 years.19,20However, in the elderly, polypharmacy, drug-drug, drug-food and drug-disease interactions, difficulty in maintaining INR at target, impaired renal function, poor nutritional status, low body weight, risk of falls, cognitive impairment and the increased risk of both stroke and bleeding, are all relatively frequent and may justify physicians’ concerns and hinder anticoagulation prescribing or the decision to prescribe cautiously resorting to infratherapeutic doses.21-23VKAs, namely warfarin, are superior to antiplatelet therapy for the prevention of stroke, with warfarin associated with a reduction of 52% in the risk of fatal or disabling stroke and intracranial haemorrhage (ICH) compared with aspirin 75mg daily in patients ≥75 years.24,25On the other hand, NOACs have an improved efficacy/safety ratio, fewer food and drug interactions compared to VKAs and do not need routine blood level monitoring due to their predicta-ble effect. Studies have shown that OAC are safe and effective in very elderly AF patients (≥85 years) and that NOACs have lower mortality rates and similar major bleeding risk compared to VKAs.22,23
NOACs were the most commonly preferable drugs (62.8%), with apixaban and rivaroxaban being the most used. However, 37.2% of patients were on VKAs, with warfarin prescribed in nearly all cases. Since the introduction of NOACs, an increasing number of newly diagnosed AF patients have been treated with these drugs instead of VKAs. However, stroke prevention strategies around countries are markedly heterogeneous and NOACs are frequently used with an individualized approach instead of according to stroke risk scores and guidelines, with an overuse in patients with a lower stroke risk, in the elderly and with a reported underuse in high risk stroke patients.26-28In our study, the high percentage of warfarin use could be explained by the low cost of it compared to NOACs and by some physicians concerns about the limited availability of the antidote and lastly also concerns around the difficulty of assessing efficacy through analytical tests. In the national context, it is also worth referring to the potential role of prescribing indicators, against which performance is measured, which call f or the use of VKAs in detriment of NOACs.29
Moreover, a quarter of the sample was incorrectly anticoagulated. Most patients were considered incorrectly anticoagulated and the main reason was being on an infra-therapeutic dose. Such prescribing pattern is likely to result from physician’s concerns about the bleeding risk and renal function. For patients prescribed resorting to a supratherapeutic dose, the prescribing behaviour is likely to result from concerns about inadequate follow-up without regular ability to monitor renal function and INR.
This study has some limitations that need to be acknowledged. First, our sample is not representative of the population and the sample achieved was considerably lower than the estimate, implying an error adjusted to 3% and thus not enabling extrapolation of findings. Second, we have only used one value of INR to define if a patient was correctly or incorrectly anticoagulated. This may introduce some misclassification bias, because the last value could be on therapeutic range, but the rest of the values out. Therefore, a better way would have been to consider the time in therapeutic range (TTR). However, we did not have access to the patient’s diaries of INR, so TTR could not be estimated. Finally, medication adherence was not considered and was in fact beyond the scope of the study. Notwithstanding, this is an important feature which may impact deeply on potential treatment outcomes and should as such be considered in future research.
ConclusionData suggest a high proportion of patients eligible for OACs are not receiving treatment. Moreover, among those treated, a quarter was incorrectly anticoagulated. Age and concurrent medication seem to be important predictors for different prescribing patterns. Given the evidence of improved safety performance of OAC, namely of NOACs, and a favourable risk-benefit ratio for AF patients, regardless of age, there is no justification for withholding, ending or not initiating OAC. The institution of compulsory continuous professional deve-lopment or health-system performance indicators should be considered to improve the prescribing pattern.