Non-invasive ventilation (NIV) is a safe ventilatory strategy to improve hypoxemia in patients with multiple causes of acute respiratory failure (ARF).1The major advantage associated with NIV lies in its ability to override the need for intubation, thereby reducing complications associated with airway manipulation.2Other non-negligible additional advantages are: the absence of need for sedation (and eventual curarization); the ability to maintain neurological monitoring and the lower risk of barotrauma.
The use of NIV is particularly well studied and recommended in ARF secondary to exacerbation of Chronic Obstructive Pulmonary Disease (COPD) and cardiogenic pulmonary oedema/decompensated heart failure. Although with less evidence, its benefit is still recognized in addressing postoperative respiratory failure, in managing ARF in immunocompromised patients and as prevention of post-extubation hypercapnia in high-risk patients.1
Current evidence suggests caution in the use of NIV as the preferred initial ventilatory method inde novohypoxemic ARF due to the high failure rates. This is related to the delay in identifying signs of NIV failure and the consequent delay in invasive mechanical ventilation (IMV), which is associated with increased morbidity and mortality.3Accordingly, the latest guidelines of recognized societies- European Respiratory Society (ERS) and American Thoracic Society (ATS) - do not provide recommendation on the use of NIV inde novoARF (in favour or against).1
More recent data points to the possible advantage of using NIV in a controlled manner in patients withde novoARF, highlighting higher success rates.4However, most studies have a strict population sample, which compromises the external validation of the evidence.3,5,6Therefore the role of NIV inde novoARF remains undefined and, although often used in this context, hypoxemic ARF remains an informal indication for NIV.
The present study aims to evaluate the use of NIV in the group ofde novoARF patients treated at an Intensive Care Unit (ICU), to identify success predictors of its use, and to define groups in which NIV can prevent IMV.
METHODS
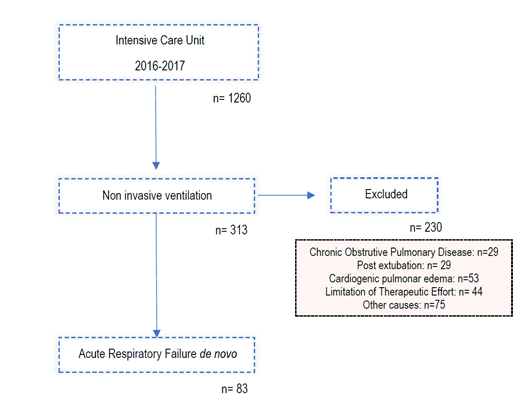
A longitudinal retrospective study was conducted involving patients admitted to the Centro Hospitalar de Trás-os-Montes e Alto Intensive Care Unit of Douro from 2016 to 2017 in which non-invasive ventilation was initiated during newly diagnosed acute respiratory failure (PaO2 / FiO2 < 200). Patients with community acquired pneumonia (CAP) and acute respiratory distress syndrome (ARDS), according to Berlin criteria, were included. Due to the retrospective character of this study, no ethics approval was required for the presentation of this paper.
Data were collected through consultation of clinical files (medical and nursing files). In addition, we also obtained data from the records of a recently implemented protocol in the unit on the use of NIV. This protocol records hourly clinical data (including respiratory rate), as well as evolutionary gas data from all patients on NIV.
As can be seen in image one, we excluded patients with:
1. Respiratory failure due to COPD exacerbation
2. Respiratory failure due to cardiogenic pulmonary oedema
3. Respiratory failure in immunocompromised patients
4. Post-extubation respiratory failure
5. Respiratory failure as a consequence of trauma
6. Therapeutic effort limitation (TEL).
Response to NIV introduction was considered when patients fulfilled all of the following clinical and gasometric criteria:
1. pH normalization (in patients with abnormal arterial pH on admission) and
2. Decreased respiratory effort with respiratory rates (RR) less than 25 cycles per minute and
3. Improvement of PaO2 / FiO2 ratio to greater than 200
None of the patients that fulfilled all of the above criteria needed to be invasively ventilated nor died. These patients were included in the “Responders” group. Patients who failed to meet all the above clinical and gasometric criteria of response to NIV application were intubated and invasively ventilated and included in the “Non-responders” group.
Due to the retrospective nature of our study, the group of work decided not to define a limited time to response, as we also included patients with late response to NIV (after 24 hours on NIV).
We compared the “Responders” group with patients who failed to respond, according to demographic data, blood gas and severity scores at admission. The used severity scores were the “Acute physiology and chronic health evaluation (APACHE) II”,7 the “Simplified Acute Physiology Score (SAPS) II”8 and the “Sequential Organ Failure Assessment (SOFA) Score”.9
We focus on the comparison of groups with regard to clinical and gasimetric findings in the first two hours after initiation of NIV, with the ultimate goal of trying to identify factors for early prediction of NIV success.
Statistical treatment of data was performed using the IBM SPSS v23 program. Quantitative variables with normal distribution were described as means and standard deviations. For quantitative variables with non-normal distribution, we used the median and interquartile distance. Qualitative variables were reported as absolute number and percentages. Student’s t-test was used to compare means amongst groups of independent samples. For variables with non-normal distribution, the corresponding nonparametric version of the above-mentioned test was used: Mann-Whitney U. The normality and heteroscedasticity of the variables were studied using the histogram (with its privilege), the Kolmogrov-Smirnov test and the Levene test. Hypothesis tests were considered significant whenever the respective test value (pvalue) did not exceed the significance level of 5%.
RESULTS
From 2016 to 2017, 83 patients withde novoARF were treated with NIV. Regarding demographic data (Table 1), 48 patients (57.8%) were male and 35 (42.2%) female, with a median age of 67 ± 22. The sample was composed mainly by medical admissions (96.4%) although 3 patients had ARF in the postoperative context. In terms of severity scores on admission, the sample presented an average APACHE score of 19.8 ± 7.2, median SAPS II score of 42 ± 17, and a median SOFA score of 7 ± 5. The most common comorbidity was heart failure, identified in 40.9% of the patients, followed by chronic liver disease (22.9%), diabetes mellitus (20.5%), COPD (19.3%) and arrhythmogenic heart disease (18.1%). The preferential ventilation mode was Continuous Positive Airway Pressure (CPAP), used in 61% of the cases, in agreement with the main indication for NIV in these patients - isolated hypoxemia with PaO2/ FiO2 ratio <200, without hypercapnia or respiratory acidosis. In 75 of the 83 patients (90.4%) NIV was introduced in the ICU, 42.7% of them (35 patients) started the ventilatory support within the first hour. Overall, NIV succeeded in 50 patients (60%). The most common aetiology of ARF was the CAP (bacterial or viral) where the success rate approached 70%. Of the 20 secondary ARDS patients, 14 represented therapeutic failure (70%) requiring rescue IMV. Of note we had a high prevalence of secondary ARDS caused by acute necrotizing pancreatitis, 7 out of 20 patients (35%) all of them failed to respond to NIV.
In 12 out of 50 successfully treated patients (24%), the timing to response was only one hour. However, a significant proportion of patients (21/42%), whose NIV introduction was considered successful, showed late total response (i.e. after 24 hours).
Table 1: Sample general characterization.
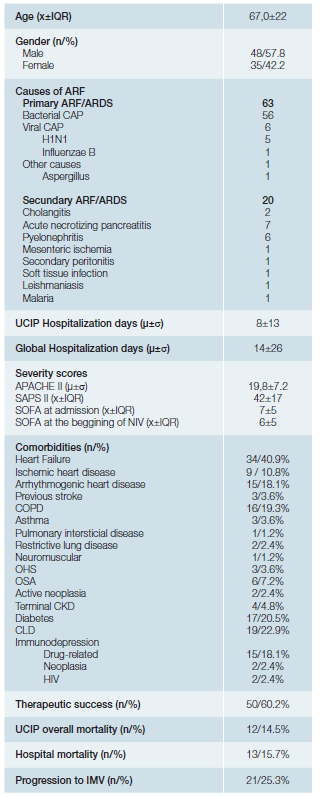
COPD: chronic obstructive pulmonary disease; OHS: obesity hypoventilation syndrome; OSA: obstructive sleep apnea syndrome; CKD: chronic kidney disease; CLD: chronic liver disease; HIV: human immunodeficiency virus. X= median; μ= mean; IQR= interquartil range; σ= standard deviation; n= absolute number;
Patients who succeeded presented significantly lower pre-NIV severity scores (APACHE II of 17.5versus22; SAPS II of 37.5versus48; SOFA score of 6versus9;p <0.001). Therapeutic success was independent of gender (p = 0.969), age (median age of 64 in the unsuccessful treated groupversus67 in the group with response to NIV introductionp = 0.773), type of admission (medical versus surgical,p = 0.560) and most of the comorbidities, as shown in table 2. One should emphasize that these findings, particularly those related to age and gender, made the groups (responders versus non-responders) comparable. Nevertheless, it must be noted that patients with favourable response to NIV introduction, had a significantly higher rate of heart failure as comorbidity (50% in opposition to 27.3% in the failing group,p = 0.039). Table 3 (A and B) shows the blood gas variables of the groups in comparison at time 0 (moment of introduction of NIV) and at time 2 (2 hours after the onset of the technique). Table 3C shows the differences in the blood gas variables between the time of admission and two hours after beginning ventilatory technique, in an attempt to find predictors of success for NIV use through gasometric changes.
Table 2: Sample general characterization - Comparision responders with non-responders.
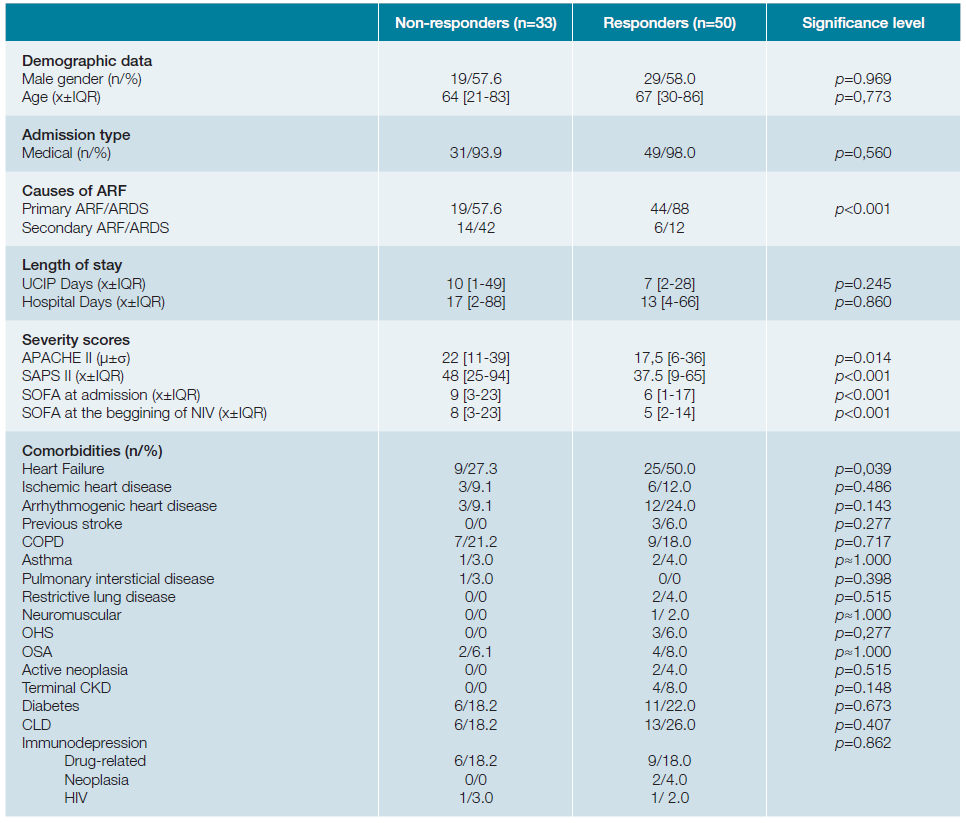
COPD: chronic obstructive pulmonary disease; OHS: obesity hypoventilation syndrome; OSA: obstructive sleep apnea syndrome; CKD: chronic kidney disease; CLD: chronic liver disease; HIV: human immunodeficiency virus. X= median; μ= mean; IQR= interquartil range; σ = standard deviation; n= absolute number;
Table 3B: Comparision of clinical and gasometric variables between the groups. Variables on time 2 (two hours after introduction of NIV).
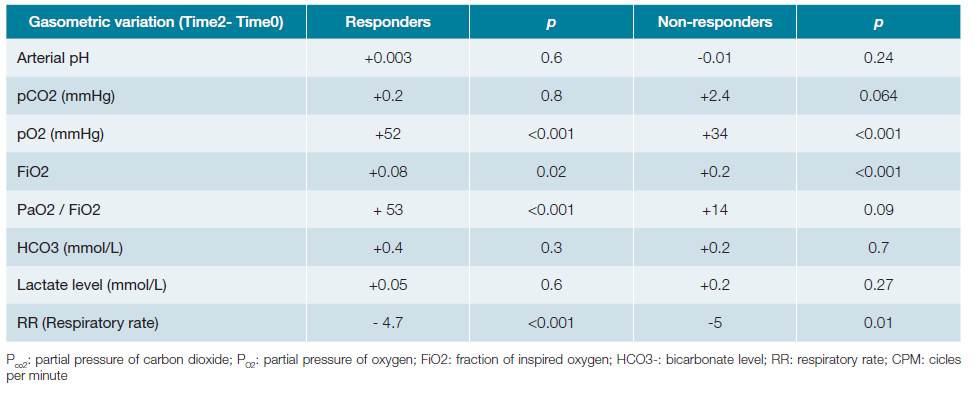
Patients who failed to respond to NIV presented significantly lower pre-treatment values in arterial pH (7.35vs7.42;p <0.001) as well as PaO2/FiO2 ratio (118vs146,p = 0.03). Concomitantly, they presented significantly higher serum lactate values (2.2 mmol/L vs 1.46 mmol/L,p <0.001) as well as higher need for vasopressor support (51.5%vs30%,p = 0.04). In contrast, PaCO2 was not significantly different between groups (41.3 mmHg in the success groupversus43.2 mmHg in the NIV failed group,p = 0.60). Two hours after the beginning of the technique, those differences remained.
Regarding the variation of clinical findings and blood gas variables after NIV introduction (difference between time variable 2 and time variable 0), the PaO2/FiO2 ratio showed a significantly higher increase in the group that favourably responded to NIV, compared to the other (+53,p <0.01 vs+14,p = 0.09). On the contrary, the variation of PaO2 (+52,p <0.001, in the success groupvs+34,p <0.001 in the failed group) and the respiratory rate (RR) (-4.7,p <0.001 vs- 5.0,p = 0.01) had similar behaviour among the groups, increasing and decreasing, respectively. The arterial pH value (success +0.003,p = 0.6; non-response group -0.01,p = 0.24), the levels of serum bicarbonate (success +0.4,p = 0.3; failure +0.2,p = 0.7) and serum lactate levels (success +0.05,p = 0.6; failure +0.2,p = 0.27) did not change significantly between the moments evaluated in none of the groups above. The remaining blood gas and respective variables variation in first two hours are identified in the Table 3C.
DISCUSSION
In our sample, NIV avoided IMV in 60% of patients with de novo ARF criteria. This finding is in accordance to a recent observational study which showed a 62% to 68% success rate in the treatment of pneumonia with non-invasive positive pressure, depending on the ventilator mode.4 However, 60.2% of this study’s sample had a do not ressucitate (DNR) decision made prior to the start of NIV, thus limiting its power and applicability to the general population with indication for invasive life and organ support. In contrast, patients with a decision to withhold treatment were not included in our study (belonged to the exclusion criteria).
One of the biggest problems in regard to establishing recommendations for NIV inde novo ARF is due to the fact that most studies with positive results use a sample of highly-selected patients.1,3,5,6,10Our study attempts to bypass that limitation by presenting a wide sample of patients with varying degrees of acute severity, pre-existing comorbidities and autonomy.
One of our key-messages is that prognosis seems to be more favourable in patients with CAP and primary ARDS, with success rates up to 70%. On the other hand, secondary ARDS was only successfully treated with NIV in 30% of the patients. This difference should be confirmed in studies with a larger dimension.
In our present study, we verified an association between the probability of success with NIV and lower severity scores. On the other hand, therapeutic success was independent from age, gender, admission type and most comorbidities, a similar finding to that reported by a 2014 Spanish study on effectivity and predictors of failure of NIV.11 The association between the presence of previous HF with no pulmonary oedema of cardiogenic origin and the higher rate of success with NIV is still not completely understood (in our sample, patients who responded favourably to NIV had a significantly higher incidence of HF even if it was not the reason for establishing NIV). This association might be due to “ARF-induced Acute HF”. It is known that patients with chronic cardiomyopathy and compromised systolic function can develop myocardial ischemia or low-output HF if respiratory work increases. The added work induced by ARF might increase the heart’s energy requirements in up to 10 times. Introducing ventilatory support, even if non-invasive, could improve cardiac output secondary to decreasing respiratory work, despite the lack of cardiogenic pulmonary oedema.12
Regarding the ABGs and clinical findings 2 hours after establishing NIV, the PaO2/FiO2 ratio only seems to change significantly in the subgroup of patients who respond favourably to NIV. Thus, and in accordance to current evidence,11,13the variation in this ratio in the first 2 hours might be a good tool to decide between NIV or the need to establish IMV.
The ERS/ATS guidelines warns us that NIV is not effective in reducing respiratory work in patients with de novo ARF,1contrary to what is verified in patients with hypercapnic respiratory failure, where the ability of NIV to decrease respiratory work has been clearly demonstrated. Our study reinforces this idea once more, given that respiratory frequency did not decrease in a statistically significant fashion in patients who achieved therapeutic success with NIV. As such, hypoxia should be our main focus in this group of patients, rather than seric pH.
This study is methodologically limited. Firstly, by its retrospective nature and secondly, it would have been important to evaluate the occurrence of events such as barotrauma, given that an important proportion of patients with ARDS need high expiratory pressures to improve oxygenation. External validity might also be hampered by our high proportion of patients with diagnosed HF; its impact on the therapeutic success of NIV is not yet clear when cardiogenic pulmonary oedema is absent.
In conclusion, this group of authors believes there is a role for NIV in selected patients with de novo ARF. Patients who seem to have a higher probability of NIV success are: patients with CAP; with or without moderate ARDS, rather than severe; with no respiratory acidemia; patients with no lactacidemia or patients with no need for vasopressor therapy. Patients with HF also seemed to benefit.
The patients with de novo ARF that start NIV should be carefully monitored, since their response within the first hours can be used as a decision-making tool. Our group suggests that the delta in the PaO2/FiO2 ratio might predict response to NIV. Nonetheless, the threshold for changing ventilatory strategies is still undefined, which underscores the need for an experienced professional to tailor the therapeutic strategy to the individual patient.