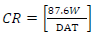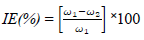Introduction
Corrosion is a process whereby materials are degraded due to their interaction with the environment. There is a deep industrial and academic interest on CI 1,2.
Corrosion is often encountered and difficult to eliminate, but it can be reduced or prevented 3.
CI are chemicals that slow down CR, when applied in small quantities to environments that cause corrosion 4.
Recently, researchers have looked into finding suitable organic inhibitors for various corrosive media 5.
These inhibitors lower the corrosion process, enhancing or changing the cathodic or anodic reaction, decreasing the reactants diffusion rate towards the metal surface, and lowering its electrical resistance 6.
The inhibitor type and C, the metal surface microstructure, IT, the solution pH and T have an effect on the contact with the surface and creation of a protective film on it 7-10.
Organic CI, such as grapefruit peels used in this research, are environmentally benign, non-toxic and inexpensive 11-16.
Grapefruit is a citrus fruit with a bittersweet or sour flavor. It contains several vitamins and minerals. It can be eaten whole, in juice or in pulp form. It may also help with weight control 17.
Ti is a naturally occurring metal with various advantageous mechanical and chemical properties. It is among the most regularly employed building materials in the aerospace sector. Ti alloys have become increasingly popular, due to their low cost, increased availability, lightweight, high strength-to-weight ratio and low elasticity modulus. It is currently widely employed in a variety of industrial applications. Paints, coatings, steel-alloying, jewelry, industrial storage tanks, building materials and automotive are just a few of Ti and its alloys applications 18.
This study examined CP as an oil-based inhibitor for preventing TS corrosion in 0.5 M HCl and 0.25 M H2SO4 media. WL and OCP techniques were used to investigate the impact of CP surfactant inhibitor. To demonstrate the IE(%) of TS corrosion by CP, Pc tests were performed, and the adsorption isotherm was studied.
Experimental methods
Oil extract from CP peels was bought from a market in Ota, Ogun State, Nigeria. The grapefruit peels were cut into pieces and dried in an oven for two days, at 150 ºC, to remove their moisture. The dried peels were blended. 800 g pulverized grape peels were placed onto a filter paper, put in a simple distillation flask, and saturated with C6H14 extractor solvent, which was added underneath the flask. After that, the flask equipment was heated to extract CP oil. C6H14 was removed after being boiled. The chemical composition test and Pc analysis of CP extracted oil were done at the Department of Chemistry, Covenant University. The acidic solutions were prepared with 200 mL 0.25 M H2SO4 and 0.5 M HCl with distilled H2O. CP was added to the acidic solutions in the following proportions: 5, 4, 3, 2 and 1 mL, respectively. TS samples were prepared and cut into 10 x 10 x 3 mm pieces. This analysis was carried out for 336 h (14 days). The sample was removed every 24 h from the acids and inhibitor mixture, washed in water and acetone, dried and weighed appropriately. XRF, at the University of Ibadan, was used for WL and polarization measurements. CR and IE(%) were calculated using the mathematical formulae in equations 1 and 2, respectively.
where A represents area (cm2), T represents IT (h), and D represents density (g/cm2). The mathematical expression was used to approximate IE(%).
where ω1 and ω2 represent WL of TS without and with CP in the electrolyte, at specific C.
Table 1 shows Pc constituents of CP.
Results and discussion
WL study and OCP
The previous report of this work recorded OCP and WL results by showing CP excellent performance. WL experiment was carried out for 336 h IT. CR and IE(%) values, for TS samples in both HCl and H2SO4 solutions with CP, were calculated. OCP analysis demonstrated that CP performed predominantly as a mixed inhibitor in both acidic solutions, thereby suppressing CR to reasonable values. The summary of the results is expressed in Table 2.
Table 2: Summary of CR, IE(%) and OCP results.
| Parameters | HCl | H2SO4 |
|---|---|---|
| CR (mm/y) | 0.0002 | 0.0403 |
| IE(%) | 91 | 77 |
| OCP | anodic reaction | cathodic reaction |
FTIR analysis
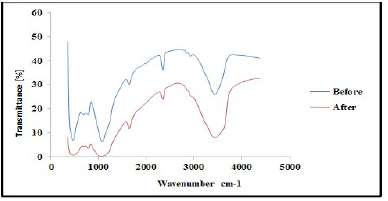
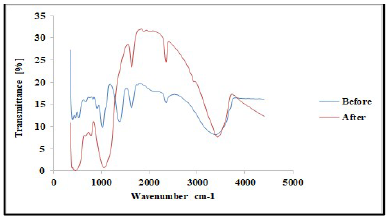
FTIR analysis was carried out to confirm the presence of various functional groups in CP organic compounds. Figs. 1 and 2 demonstrate the curves of transmission (%) and WN (cm-1), by showing the functional groups present in CP extract. For instance, some functional groups were recognized at WN different slopes, and compared with the theoretical table 19. Alkene with =C-H stretch bond, at 1250-1020 cm-1 WN, and aliphatic amines were depleted by the transmittance (%) slope reduction, with the formation of a thick film on the TS surfaces. Alcohols, carboxylic acid and esters C-O stretch bonds were due to CR retardation, as the WN moved to 1320-1000 cm-1. Also, the slope in Fig. 2 shows that, at 2695-2830 cm-1 WN, aldehydes H-C=O:C-H stretch bond was noted. At that point, the transmittance slope was reduced from 42 to 26%, confirming CP great adsorption performance. At 2830-3000 cm-1 WN, C-H stretch bond revealed alkane formation. At 3200-3500 cm-1 WN, O-H stretch, H-bonded alcohols and phenols were produced 20. Furthermore, CP molecules were actively absorbed onto the TS samples surface, and most polar functional groups strongly displaced SO4 2- and Cl- ions active corrosion agents in the solutions.
OM images
TS samples were viewed under OM, to assess chloride and H2SO4 corrosion damage effect on the TS surface, before and after corrosion, without and with 1 and 5% C of CP. Fig. 3 shows OM image of the uncorroded TS sample. TS samples had a mirror-like shining surface, which indicated no degradation.
Fig. 4 shows that most of the magnified TS sample surface was damaged by general corrosion 21.
In Fig. 5, it is seen that the entire TS surface has pitting corrosion, which suggests damage by SO4 2- ion 22.
Figs. 6 and 7 show that CP protected the TS surface from general and pitting corrosion. CP created a film that covered the TS surface (Fig. 6), hindering Cl- ion attack 23,24. Similarly, the TS sample (Fig. 7) was protected from SO4 2- corrosion damage, as CP formed a thin barrier on its surface, through a strong adsorption effect. This confirms the strong corrosion IE(%) of CP in acidic media.
Conclusion
The adsorption study on CP revealed that it is a useful organic CI for TS corrosion in HCl and H2SO4 acidic solutions. The electrochemical technique confirmed that, as the C of CP increased, TS samples were protected from corrosion damage, at every C of the acidic solutions. Also, CP antioxidant reactors, known as active components, were the main factors responsible for its excellent IE(%), by reducing corrosion damage on TS samples in both acidic media. Furthermore, OCP revealed that CP behaved predominantly as a mixed-type inhibitor. FTIR slopes depicted the numerous functional groups present in CP, such as O-H stretch, H- bonded alcohols, phenols and others. Those functional groups enabled CP IE(%), by suppressing TS corrosion damage in the acidic solutions. Furthermore, OM revealed the corrosive media and CP IE(%) effects on the TS surface morphology, thereby confirming the inhibitor strong adsorption onto the alloy samples surface.
Acknowledgement
The authors appreciate Covenant University for its financial support towards the completion of this research work.
Conflict of interest statement
The authors declare that they have no competing financial or personal interests that could influence this study.
Authors’ contributions
Olayemi Abosede Odunlami: conceived the work. Muyiwa Fajobi: design the work. Toluwalase Fernandez: wrote the paper. Okonkwo Victor: wrote the paper. Williams Ayobami-Ojo: reviewed the paper.
Abbreviations
C6H14: n-hexane
C: concentration
CI: corrosion inhibitor
CP: Citrus paradisi
CR: corrosion rate
FTIR: Fourier-transform infrared spectroscopy
H2SO4: sulfuric acid
HCl: hydrochloric acid
IE(%): inhibition efficiency
IT: immersion time
OCP: open circuit potential
OM: optical macroscopy
Pc: phytochemicals
T: temperature
TS: Ti steel
WL: weight loss
WN: wavenumber
XRF: X-ray fluorescence spectroscopy