Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portugaliae Electrochimica Acta
versão impressa ISSN 0872-1904
Port. Electrochim. Acta v.28 n.2 Coimbra 2010
The Polarographic Reduction and Electrode Kinetics of Anti-depressant Drug Bupropion Hydrochloride
Sharda Samota, Ashish Garg, Rajayashree Pandey*
Department of Chemistry, University of Rajasthan, Jaipur, India
DOI: 10.4152/pea.201002087
Abstract
The electroreduction of the antidepressant drug Bupropion hydrochloride has been studied in aqueous media at dme. Single well defined wave was obtained in different supporting electrolytes, like, KNO3, ammonium citrate buffer, acetate buffer, B. R. buffer, etc. The effect of pH on this reduction has been studied in B. R. buffer in the pH range 3.13 – 11.0. The Bupropion hydrochloride is best reduced in slightly acidic medium. This behavior was attributed to the reduction of >C=O group present in the drug. The effect of concentration and temperature on half wave potential has also been investigated. The reduction of drug was found to be irreversible and diffusion controlled, hence kinetic parameters (K0fh, αn) are evaluated using Meites Israel and Gaur Bharagav,s method. Further, with increase in temperature the values of K0fh also increase, showing that irreversibility of the system decreases on increasing the temperature.
Keywords: DC polarography, reduction, antidepressant drug, Bupropion hydrochloride.
Introduction
Bupropion (previously known as amfebutamone) is a typical antidepressant that acts as a norepinephrine and dopamine reuptake inhibitor and nicotine antagonist. It belongs to the chemical class of aminoketone and is similar in structure to the stimulant cathionone, to the anorectic diethylpropion, and to the phenethylamines in general. Initially researched and marketed as an antidepressant, Bupropion was subsequently found to be effective as a smoking cessation aid [1].
Bupropion was the first non-nicotine based therapy for smoking cessation to be approved by the Food and Drug Administration (FDA) and is still the most widely prescribed [2]. Interest in Bupropion as a smoking cessation aid began after reports of successful cessation by smokers prescribed the drug as antidepressant. However, Bupropion does not appear to work via its antidepressant effects, i.e., it is effective in those with or without current or past depressive symptoms [3-5].
Animal studies have shown that Bupropion has an effect on numerous nicotine actions. It also alters brain reward circuits influenced by nicotine, reversing the elevated intracranial self-stimulation thresholds resulting from nicotine abstinence, thus impairing the negative reinforcing effects of nicotine [6-8].
Although Bupropion’s mechanism of action is uncertain, it appears to act on a number of brain pathways involved in dependence[9]. It is known to inhibit the neuronal reuptake of dopamine in the mesolimbic system, the pathway implicated in the reinforcing effects of nicotine and noradrenaline in the locus coeruleus, which is thought to be involved in nicotine withdrawal. Bupropion also acts as an antagonist for neuronal nicotinic acetylcholine receptors [10-13].
Bupropion has formula weight 239.74 g/mol and melting point 233-234 oC. The hydrochloric salt of Bupropion is a white crystalline powder with a bitter taste; freely soluble in water, methanol, ethanol.
2-(tert-butylamino)-1-(3-chlorophenyl) propan-1-one
Various methods have been reported for the estimation of Bupropion in pharmaceutical preparations and biological fluids viz LC14, HPLC [15-16] and mass spectrometry [17]. Although chromatographic methods offer high degree of specificity, yet, sample clean up and instrument limitations preclude their use in routine clinical studies. Voltammetric technique offers another possibility for the estimation of this compound. Review of the literature revealed that, up to the present time, nothing has been reported concerning the polarographic behavior of Bupropion. The molecular structure of the studied compound is characterized by the presence of an electroactive reducible carbonyl group; this initiated the present study. The aim of the present paper is to study the electrochemical behavior of Bupropion hydrochloride at dropping mercury electrode by direct current polarography.
Experimental
Materials and reagents
A stock solution of Bupropion hydrochloride (5´10-3) was prepared in triple distilled water and further diluted with the same solvent to give appropriate concentration for the working range. KNO3 (0.5 M), acetate buffer (0.1 M), B. R. buffer (0.04 M), ammonium citrate buffer (0.2 M) were used as the supporting electrolytes. pH study was carried out in B. R. buffer in the pH range 3.13- 11.0. All solutions were prepared from Analar-grade reagents (Merck and Sigma) in triply distilled water.
Apparatus
Polarographic experiments were carried out with Elico D.C. recording polarography CL 357. The current voltage measurements were performed with three electrode assembly, a dropping mercury electrode as working electrode, calomel as reference electrode and platinum electrode as counter electrode. The current responses and applied potentials were recorded at scan rate 100 mV/min.
The dropping mercury electrode had the capillary characteristics, m = 2.768 mg/s, t = 3.0 s, h = 60 cm. pH was adjusted to suitable range by Elico digital pH meter.
Results and discussion
Electrochemical behavior of Bupropion hydrochloride has been studied in different supporting electrolytes in aqueous medium. Reduction of carbonyl group in this media gave one well-defined wave as expected. The reduction was found to be diffusion controlled (the plot of id versus concentration was found to be linear and also the temperature coefficient was found to be 1-2%/deg).
Linear plots were obtained for log i/id-i versus Ed.e. with slope 0.0591/αn Volts and zero intercept on y-axis gave the value of E1/2. The values of slopes indicated that the reduction of Bupropion hydrochloride is irreversible. The kinetic parameters were calculated from Meites-Israel method and also by the further modified Gaur-Bhargava’s method.
According to them the equation for irreversible wave was found to be
d.e. = 0.05915/αnlog 1.349Kofht1/2/D1/2 – 0.0542/αnlog i/id-i
which may be written as
Ed.e. = E1/2 – 0.0542/αnlog i/id-i
E1/2 = 0.05915/αn log 1.349Kofht1/2/D1/2
where Kofh = formal rate constant for forward reaction; D = diffusion constant; αn = transfer coefficient; and other terms have usual significance.
Thus the value of αn was obtained from the slope of the straight line corresponding to Ed.e. V/s log i/id-i. The intercept of the same plot gives the value of E1/2 which was used to calculate Kofh after getting the value of D1/2 from the Ilkovic equation. Meites Israel has extended the Koutecky’s graphical method into comparatively more precise mathematical form. Further, Gaur-Bhargava has also extended the Koutecky’s treatment for irreversible wave, since according to them the diffusion to the electrode surface (mercury drop) is spherical and not a linear one, as assumed by Meites and Israel [18].
Gaur Bhargava’s modification:-
E1/2 = 0.05915/ αn log K0fht1/2/(antilog c)D1/2
Polarogram of Bupropion hydrochloride in different supporting electrolytes
Electrochemical behavior of Bupropion hydrochloride has been studied as a function of salt concentration in different supporting electrolytes, as shown in Fig. 1:
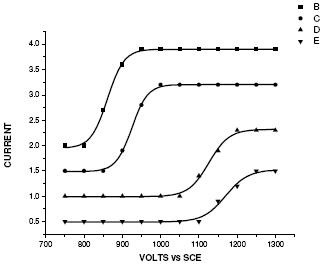
Figure 1. Current-voltage curves of Bupropion hydrochloride in various supporting electrolytes- B, C, D and E were in B. R. buffer (0.04 M), acetate buffer (0.1 M), ammonium citrate buffer (0.2 M), KNO3 (0.5 M), respectively.
From the graphs it is clear that the diffusion currents decrease with the increase in concentration of the salt. Since the diffusion current id depends on the diffusion coefficient of the electroactive species, which in turn depends on the viscosity of the solution, increasing the viscosity causes id to decrease [19].
Further, the half wave potential of the drug shifts to the more negative direction on increasing the concentration of the salt and it was found to be 0.865, 0.925, 1.105, 1.170 Volts in B. R. buffer (0.04 M), acetate buffer (0.1 M), ammonium citrate buffer (0.2 M), and KNO3 (0.5 M), respectively. This increase in potential is due to the alteration in the rate of electron transfer step for an irreversible electrode reaction [20].
Effect of concentration
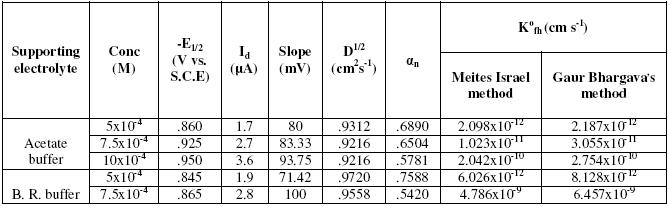
Polarograms were run of solutions containing Bupropion hydrochloride in concentrations ranging from 5´10-4 M to 10-3 in acetate buffer (pH=4.5) and B. R. buffer (pH=2.87).
The results indicate that the reaction is irreversible. The half-wave potential shifts towards more negative value with increasing the concentration. This behavior was observed in both buffers. Further, the height of the wave was found to vary directly with the concentration indicating the electrode reaction to be diffusion controlled. The values of K0fh indicate that the electrode reaction is more irreversible at low concentrations. The results are shown in Table 1.
Table 1. Effect of concentration.
Effect of pH
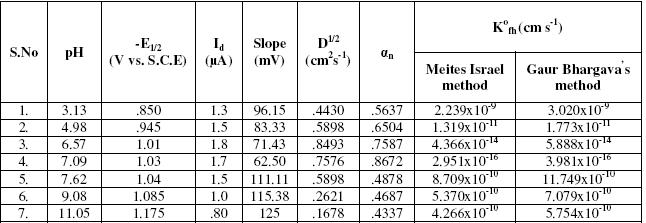
Bupropion hydrochloride was found to be reduced at dme in the pH range 3.13-11.0 in B. R. buffer. Variation of half wave potential and diffusion current with pH shows the involvement of proton in the electrode reaction [21]. As the pH of the solution increases, the wave height increases up to pH 6.57, than its height begins to decrease. In highly alkaline solution an ill defined wave was observed which suggests that the drug was best reduced in slightly acidic medium.
The half wave potential shift to more negative direction on increasing the pH, showing that the reduction becomes difficult at higher pH. Linear plots were obtained for log i/id-i versus Ed.e. at every pH value. Assuming that the rate-determining step involves the transfer of two electrons, the value of the slope denotes that the reduction process is irreversible. From the values of K0fh, listed in Table 2, it is clear that the degree of irreversibility increases as the pH value increases up to pH 7, after which it decreases again.
Table 2. Effect of pH.
The number of protons, involved in the reduction process for each pH value was determined using the relation [22-23]:
ΔE1/2/ΔpH = 0.05915/αn(ZH+)
where αn is the transfer coefficient. The value of αn is calculated from the following equation
E = E1/2 – (0.05915/ αn) log i/id-i
where id is the diffusion current.
The ZH+ was found in between 0.18 to 0.41 at different pH values, i.e., one proton is probably consumed in the rate-determining step of the electrode reaction. This is further confirmed by the plot of E1/2 = f(pH); slope value is more nearly consistent with one proton involvement (-30 to -60 mV/pH) than two (-60 to -120 mV/pH) [24].
![]()
Effect of temperature
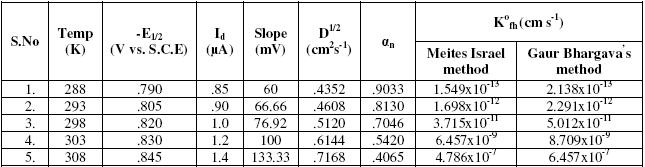
Titled drug exhibits single well defined reduction wave at all the temperatures ranging from 15 oC to 35 oC. Temperature dependence on the diffusion current for the reduction wave was found to be linear. The temperature coefficient of the diffusion current, 1% to 4% per degree, is precisely that to be associated with diffusion controlled process. Since the temperature coefficients of the diffusion currents of most organic molecules are usually of the order 1% to 2%/deg [25]. The temperature coefficients of the half wave potentials were 15-10 mV per degree, indicating the irreversible electrode reaction [26]. The results are shown in Table 3.
Table 3. Effect of temperature.
Conclusion
A simple and sensitive method was developed for the qualitative determination of Bupropion hydrochloride. The wave given by the titled drug was found to be diffusion controlled since its height is proportional to concentration, varies with concentration and nature of the supporting electrolyte, and its temperature coefficient was found to be 1-2%/deg.
The behavior of the half wave potential shows that the reaction is irreversible. While the half wave potential is pH dependent, as one would expect for an organic reaction, the shift per pH unit exceeds the theoretical values predicted for reversible reactions. In addition, the half wave potential is not independent of concentration, but apparently varies with its logarithmic value. pH study reveals that drug can be best reduced in slightly acidic medium. Further, the values of K0fh increase with increase in temperature, which suggests that irreversibility of the system decreases with increase in temperature; this implies that the reduction product of the drug is stable at lower temperature. Provided that proper attention is given to the factors of pH, concentration, temperature, etc., on the half-wave potential, the wave could be of value for qualitative identification.
References
1. M. Siepmann, K. Werner, C. Schindler, R. Oertel, W. Kirch, Psychopharmacology 182 (2005) 597-598. [10.1007/s00213-005-0128-y]
2. V.C. Wing, M. Shoaib, Psychopharmacology 195 (2007) 303-313. 10.1007/s00213-007-0902-0
3. R.D. Hurt, D.P. Sachs, E.D. Glover, K.P. Offord, J.A. Johnston, L.C. Dale, M.A. Khayrallah, D.R. Schroeder, P.N. Glover, C.R. Sullivan, I.T. Croghan, P.M. Sullivan, N. Engl. J. Med. 337 (1997) 1195-1202.
4. M.G. Goldstein, J. Clin. Psychiatry 4 (1998) 66-72.
5. K.E. Hayford, C.A. Patten, T.A. Rummans, D.R. Schroeder, K.P. Offord, I.T. Crogham, E.D. Glover, D.P. Sachs, R.D. Hurt, Br. J. Psychiatry 174 (1999) 173-178.
6. J.F. Cryan, A.W. Bruijnzeel, K.L. Skjei, A. Markou, Psychopharmacology 168 (2003) 347-358. [10.1007/s00213-003-1445-7]
7. M.P. Epping-Jordan, S.S. Watkins, G.F. Koob, A. Markou, Nature 393 (1998) 76-79. [10.1038/30001]
8. J.E. Slemmer, B.R. Martin, M.I. Damaj, J. Pharmacol. Exp. Ther. 295 (2000) 321-327.
9. C. Warner, M. Shoaib, Addict. Biol. 10 (2005) 219-231. [10.1080/13556210500222670]
10. J.A. Ascher, J.O. Cole, L.N. Colin, J.P. Feighner, R.M. Ferris, H.C. Fibiger, R.N. Golden, P. Martin, W.Z. Potter, E. Richelson et al., J. Clin. Psychiatry 56 (1995) 395-401.
11. W.A. Corrigal, K.B. Franklin, K.M. Coen, P.B. Clarke, Psychopharmacology 107 (1992) 285-289. [10.1007/BF02245149]
12. R.M. Ferris, B.R. Cooper, R.A. Maxwell, J. Clin. Psychiatry 4 (1983) 74-78.
13. L.H. Ferry, Prim. Care 26 (1999) 653-69.
14. R. Coles, E.D. Kharasch, J. Chromatography B 857 (2007) 67-75. [10.1016/j.jchromb.2007.07.007]
15. M. Qi, P. Wang, Y. Geng, J. Gu, R. Fu, J. Chinese Pharma. Sci. 11 (2002) 16-18.
16. T.A. Jennison, P. Brown, J. Crossett, M.F. Urry, J. Analytical Toxicology 19 (1995) 69-72.
17. F. Badoud, E. Grata, L. Perrenoud, L. Avois, M. Saugy, S. Rudag, J.L. Veuthey, J. Chromatography, A 1216 (2009) 4423-4433. [10.1016/j.chroma.2009.03.033]
18. L. Meites, Y. Isral, J. Am. Chem. Soc. 83 (1961) 4903-4906. [10.1021/ja01485a008]
19. L. Meites, Polarographic techniques, Brooklyn, New York, 1964, p.141.
20. L. Meites, Polarographic techniques, Brooklyn, New York, 1964, p.291.
21. N.T. Abdel Ghani, M.A. El-Ries, M.A. EL-Shall, Anal. Sci. 23 (2007) 1053-1058. [10.2116/analsci.23.1053]
22. A.A. AI-Majed, F. Belal, A. Abadi, A.M.-Al-obaid, Il Farmaco 55 (2000) 233-238. [10.1016/S0014-827X(00)00009-4]
23. N. El-Enany, A. Ei-Brashy, F. Belal, N. El-Bahay, Port. Electrochim. Acta 27 (2009) 113-125. [10.4152/pea.200902113] [ Links ]
24. P. Zuman, J. Solid State Electrochem. 10 (2006) 841-851. [10.1007/s10008-006-0177-0]
25. L. Meites, Polarographic techniques, Brooklyn, New York, 1964, p.139-140.
26. L. Meites, Polarographic techniques, Brooklyn, New York, 1964, p.288.
Received 29 July 2009; accepted 10 May 2010
* Corresponding author: sharda033@yahoo.com














