Introduction
Hearing is a fundamental perception for the normal bio-psycho-social development of a child.1-2 It depends on the functioning of the auditory system, which consists of the ears and the auditory pathway.2 The ear ensures the capture of sound and its conversion into an electrical signal that travels along the auditory pathway to the auditory cortex, where it is integrated and interpreted.1-3 Because the functionality of the auditory system is known from birth, several assessment methods based on electrophysiological measurements have been developed to provide a reliable estimate of hearing in newborns and infants.1),(4-6
Since permanent hearing loss affects 1-2 per 1000 newborns, a Universal Newborn Hearing Screening (UNHS) program has been developed to diagnose these cases.1),(4-5 In Portugal, the first national guidelines of the Group for Screening and Intervention of Child Deafness (GRISI) were published in 2007, with the aim of prioritizing screening, diagnosis, and intervention for hearing loss at the national level.5),(7 Since then, the UNHS program has been widely disseminated in the Portuguese National Health Service.8
There are sensitive periods during which the central auditory pathway undergoes significant neuroplasticity, and critical time windows for audiological intervention have been defined to ensure adequate development of the central auditory pathway.2 Accordingly, all newborns must be screened in the first month of life to diagnose hearing loss by three months of age and initiate early intervention by six months of age.4-5 These timelines allow for normal language development.1),(3-4 However, recent international recommendations suggest a more ambitious goal for hospitals that already meet these requirements, advocating screening in the first month, diagnosis by two months, and intervention by three months.4
Currently, there are two validated electrophysiologic techniques for screening this population: otoacoustic emissions (OAE) and automated auditory brainstem response (AABR).4,5 OAE assesses a smaller portion of the auditory system because it only detects changes in the external auditory canal (EAC), middle ear, and inner ear up to the outer hair cells, unlike AABR, which also examines the auditory pathway.2-4),(6
Hearing loss can be classified as conductive hearing loss (CHL), sensorineural hearing loss (SNHL), or mixed hearing loss (MHL). Severity can be mild, moderate, severe, or profound.1-4 Several factors are known to increase the risk of congenital, progressive, or late-onset hearing loss. These are called risk factors and, when present, warrant a differential screening protocol consisting of a high-risk screening. These children require audiologic diagnostic evaluation even if they pass the initial screening.4
The aims of this study were to (i) estimate the incidence of SNHL in the Baixo Vouga region; (ii) determine whether the UNHS program of Centro Hospitalar do Baixo Vouga (CHBV) meets the GRISI quality criteria; (iii) assess the importance of first-degree parental consanguinity as a risk factor for hearing loss in the UNHS program; (iv) assess children’s age at screening and diagnosis; (v) identify the main difficulties in implementing the hearing screening protocol; and (vi) identify areas for protocol improvement.
Material and methods
This was a retrospective incidence study of all infants born between January 2014 and December 2018 in the Baixo Vouga region, specifically at CHBV, and at other private maternity hospitals in the region without hearing screening available. Electronic clinical records from CHBV UNHS computer platform were reviewed, and demographic and clinical data were collected, including gestational age, mode of delivery, gender, birth weight, risk factors, hearing screening results, diagnostic results (when available), and age at screening and diagnosis. For children born in private hospitals, only data on gender, risk factors, hearing screening results, diagnostic results, and age at screening or diagnosis could be collected. In these cases, missing data were assumed for the remaining variables.
Risk factors included those recommended by GRISI and first-degree parental consanguinity, as a significant number of cases of hearing loss have been identified in this context in previous years (Table 1).
Table 1 Risk factors for childhood hearing loss included in the protocol
| Risk factors for childhood hearing loss |
| Family history of childhood hearing loss |
| First-degree parental consanguinity |
| ≤32 weeks gestational age |
| Very low birth weight (< 1500g) |
| 1-minute Apgar score ≤4 or 5-minute Apgar score ≤6 |
| Craniofacial malformations or others associated with hearing loss |
| In utero infections (toxoplasmosis, rubella, cytomegalovirus, herpes, syphilis) |
| Sepsis/neonatal meningitis and/or ototoxic drugs for ≥5 days |
| Hyperbilirubinemia with exchange transfusion criteria |
| Intracranial hemorrhage |
| Invasive ventilation and neonatal intensive care for more than 48 hours |
Screening results were classified as pass (if the test result was normal) or refer (if the test did not meet the required criteria). Pass and refer thresholds were defined according to the manufacturer’s rules. A Natus®MADSEN AccuScreen device was used for OAE and AABR. Diagnostic audiologic evaluation included clinical evaluation by an otolaryngologist and auditory brainstem response (ABR) using the Interacoustics Eclipse EP25 device from Interacoustics®. All screening and diagnostic testing was performed by an audiologist with pediatric experience in a controlled sound environment, preferably during spontaneous sleep.
Depending on the presence of risk factors, each infant was assigned to one of two groups: with or without risk factors. The pediatricians were responsible for identifying these risk factors. All infants underwent an initial hearing screening, preferably between 24 and 48 hours of life, using OAE (mostly) and/or AABR. Those classified as refer performed a second screening, also with OAE and/or AABR, usually by 15 days of life. All screening tests included bilateral evaluation, regardless of the differential result of the first test. Children without risk factors and classified as pass in either the first or second screening were discharged from the protocol. Otherwise, those classified as refer in both phases or in the first phase and who did not undergo the second evaluation were sent for diagnostic audiologic evaluation, which included an otolaryngologic evaluation and diagnostic testing using the auditory brainstem response method. Infants with risk factors despite screening results were also referred for diagnostic evaluation.
For descriptive analysis, mean and standard deviation were used to characterize normally distributed quantitative variables, and median and interquartile range (IQR) were used to characterize non-normally distributed quantitative variables. Normality was determined after analysis of each histogram. Qualitative variables were expressed as absolute numbers and relative frequencies. Microsoft Excel® was used for computer data entry, and IBM® SPSS® Statistics software, version 27.0, for Mac® was used for statistical analysis.
Results
During the five-year period considered, 8,727 infants were included in the CHBV UNHS computer platform, of whom 97.55% were born in CHBV and the remaining outside the hospital center. In this population, 90.88% of infants had no risk factors, 9.07% had one or more risk factors, and the presence of risk factors could not be determined in 0.05% of infants (n=4). Among children with risk factors, 81.94% had only one risk factor, 14.27% had two risk factors, and the remaining 3.79% had three or four risk factors. The three most common risk factors were a family history of childhood hearing loss (n=263), followed by neonatal sepsis/meningitis and/or ototoxic drug administration for five or more days (n=220), and a history of first-degree parental consanguinity (n=115).
Of all children included in the platform, only 19 (0.22%) did not undergo any type of screening or diagnostic evaluation. Of the children born at CHBV, 99.86% underwent a hearing screening.
In the group without risk factors (7,931 cases), 99.87% were screened, of which 90.15% had a pass result and 9.85% had a refer result. Infants classified as refer had a second screening and the vast majority (91.15%) were discharged because they were classified as pass. Of the 69 infants referred for diagnostic evaluation, 23.18% had no evaluation, 28.99% had normal hearing, 18.84% had CHL, and 28.99% had SNHL.
In the group with risk factors (792 cases), 98.99% had an initial screening and 86.10% were classified as pass and 13.90% as refer. The referrals were sent for a second screening and 63.30% were classified as pass. Among the children with risk factors who passed the screening, 47.58% did not complete or did not undergo a diagnostic evaluation, and this percentage decreased to 27.50% in the group of children who were classified as refer in one or both screening phases.
Of all children who underwent diagnostic evaluation (474 cases), 71.73% had a normal result. Of the remaining 134 cases, 76.87% had CHL, 20.90% had SNHL, and 2.24% had MHL. For SNHL, the incidence rate was 2.4 per 1000 infants without risk factors and 27.6 per 1000 infants with risk factors. In addition, a false positive rate of 0.34% and a referral rate to an otolaryngologist of 1.24% were identified.
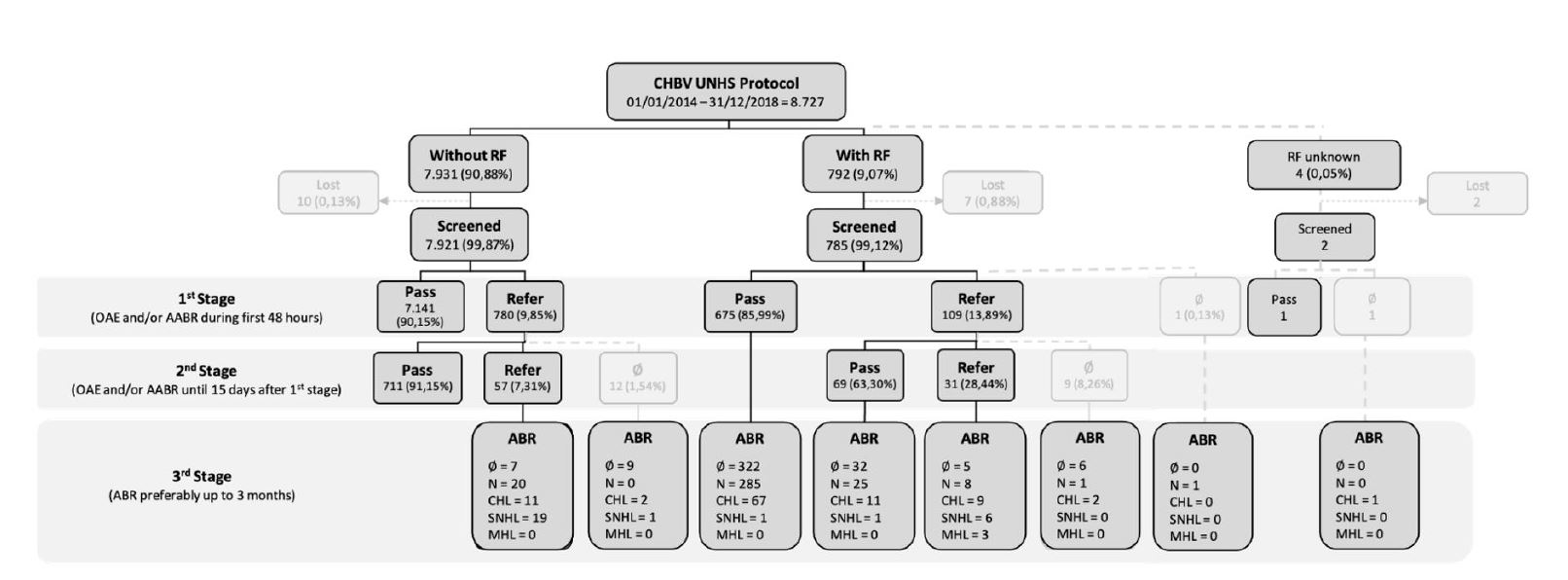
Figure 1 Results of the universal newborn hearing screening protocol. AABR: automated auditory brainstem response; ABR: auditory brainstem response; CHL: conductive hearing loss; MHL: mixed hearing loss; N: normal; OAE: otoacoustic emissions; RF: risk factors; SNHL: sensorineural hearing loss; UNHS: universal newborn hearing screening; ∅: unrealized
Table 2 Characteristics of hearing loss cases in the study
| Risk factors | Type of hearing loss | ||||
| Sensorineural hearing loss (SNHL) n=28 | Mixed hearing loss (MHL) n=3 | ||||
| Absent n=20 | 1st OAE | Refer | Refer | - | |
| 2nd OAE | Refer | - | - | ||
| ABR | SNHL (n=19) | SNHL (n=1) | - | ||
| Present n=11 | 1st AABR | Pass | Refer | Refer | Refer |
| 2nd AABR | - | Pass | Refer | Refer | |
| ABR | SNHL (n=1) | SNHL (n=1) | SNHL (n=6) | MHL (n=3) | |
| Risk factor | Preterm (n=1) | Consanguinity (n=1) | Sepsis (n=2) Family history (FH) (n=2) Consanguinity and FH (n=1) Craniofacial malformation (n=1) | Consanguinity (n=1) Consanguinity and FH (n=1) Craniofacial malformation (n=1) | |
AABR: automated auditory brainstem response; ABR: auditory brainstem response; FH: family history; MHL: mixed hearing loss; OAE: otoacoustic emissions; SNHL: sensorineural hearing loss; -: unfulfilled.
Table 2 shows the cases of hearing loss according to the presence of risk factors. Analysis of SNHL cases shows that the most common risk factors were first-degree parental consanguinity (n=4) and family history (n=4).
Children had a median age of two days (IQR 3) at the first screening and 22 days (IQR 16) at the second screening. At the time of diagnosis, children classified as refer at both screenings had a median age of 197 days (IQR 265.25), and at the time of diagnosis, children with an indication for this evaluation had a median age of 263 days (IQR 229.75).
Analysis of the rate of missed screenings showed that only 0.24% of infants (n=21) missed the first screening and 2.36% (n=21) missed the second screening. In the diagnostic phase, 381 of 855 infants (44.56%) missed or did not complete the diagnostic evaluation, of whom 92.91% had been classified as pass at either the first or second screening. When the results were broken down by year, a significant proportion of the 381 infants were born in 2018 (38% in the group classified as pass and 48% in the group classified as refer). Conversely, between 2014 and 2017, the annual rate of missed screenings at this stage varied between 10% and 24% for children classified as pass and between 8% and 15% for those classified as refer.
In infants without risk factors, screening was performed with OAE only, so it was impossible to exclude cases of auditory neuropathy. In infants with risk factors, both OAE and AABR were performed in 126 cases. Only four of these had discrepancies between the results, and all had normal ABR results. This suggests that there were no cases of auditory neuropathy in this group.
Discussion
CHBV offers newborn hearing screening to all infants born in its area of influence. This is not only an advantage for the local population, but also provides more reliable data on hearing screening and diagnosis of hearing loss in the Baixo Vouga region.
A UNHS protocol that stratifies children according to the presence or absence of risk factors for hearing loss, rather than according to their origin (nursery vs. neonatal intensive care), requires the analysis and systematic recording of risk factors in all infants. On the other hand, there may be cases where parents are unaware of the presence of risk factors at birth but recognize them later (e.g., cases of family history of hearing loss)4, and registering them on a computer platform allows correction of the child’s category and subsequent inclusion in the appropriate follow-up protocol.
Compared to other national studies, a higher prevalence of children with risk factors for hearing loss was found in this cohort, which may be due to the fact that consanguinity was considered as a risk factor and is a predominant one (one of the three most common risk factors).9-11 Most cases of consanguinity were found in children from the Romani community. This may be explained by the prevalence of people from this community in the population supported by CHBV, as evidenced by the National Study of Romani Communities, which estimates that Aveiro district has the third highest number of resident Romani people at national level, and that the Baixo Vouga region is the group of municipalities with the fourth largest Romani population.12
Regarding screening results, the rate of children classified as pass and refer was similar to that reported in other national studies.9-11 The UNHS program of CHBV was shown to meet national guidelines for quality screening, with a screening effectiveness of 99.86% (>95%), a false-positive rate of 0.34% (<3%), and a referral rate for otolaryngologic consultation of 1.24% (<4%). The results of this UNHS program were also similar to those of international programs (Table 3).13 It should be noted that the present study design did not allow estimation of the rate of false-negative screening results.
Table 3 Comparison of CDC and CHBV universal newborn hearing screening programs13
| Universal newborn hearing screening items | Data | |
| CDC (2018) | CHBV (2014-2018) | |
| Documented hearing screening Percent screened (screenings/births) Percent referred (referrals/screenings) | 98.3% (n = 3,681,776) 1.6% (n = 60,258) | 99.86% (n = 8,501*) 1.25% (n = 109) |
| No documented hearing screening Percent without documented screening (loss to follow-up/births) | 1.7% (n = 63,039) | 0.14% (n = 12*) |
| Documented diagnosis Percent diagnosed (diagnoses/referrals) | 64.1% (n = 38,634) | 75.23% (n = 82) |
| No documented diagnosis Percent without documented diagnosis (no documented diagnoses/referrals) | 35.9% (n = 21,624) | 24.77% (n = 27) |
The diagnosis of SNHL reached 2.4 per 1000 children without risk factors and 27.6 per 1000 children with risk factors, which is in line with the incidence of hearing loss described in the literature.5 The detection of SNHL cases in children without risk factors reinforces the importance of universal screening as advocated in recent decades, as opposed to selective screening for risk groups, as initially established.4 The diagnosis of SNHL in children with risk factors and a pass result also reaffirms the need for diagnostic evaluation in this group of patients and raises increased concern about missed screening in this population.
In the present study, four out of 11 children with SNHL and risk factors had first-degree family consanguinity, which was the only risk factor in half of them. This could be explained by the fact that approximately 50% of all cases of deafness have a genetic etiology, 70% of which are non-syndromic, and 80% from these are autosomal recessive.2 Therefore, in these cases there is no family history of the disease, and parental consanguinity is an important clue to the possibility of recessive inheritance.2),(14 Furthermore, in one of these cases, the result of the first screening was refer but the result of the second screening was pass, which means that if this risk factor had not been considered, this child would have been discharged from the protocol and would not have undergone diagnostic assessment. Following these findings, this research group developed a cohort study during the same time period to evaluate the importance of defining first-degree parental consanguinity as a risk factor for childhood hearing loss. This study found that children with first-degree parental consanguinity were three times more likely to have a refer result at screening than children without risk factors.15 Although additional studies are needed, the identification of a significant number of cases of parental consanguinity among children diagnosed with SNHL in CHBV is relevant. Therefore, it seems prudent to continue to consider parental consanguinity as a risk factor for hearing loss.16
The rate of missed hearing screenings comprised (i) children who did not undergo screening (due to neonatal death, transfer to more differentiated services, or absence from consecutive appointments when it was impossible to perform screening in the maternity ward) and (ii) children who did not undergo a second screening or diagnostic evaluation when indicated (including children born in CHBV but not living in its area of influence, children who changed residence, cases who had no means of contact or did not respond after several contact attempts, and children for whom it was impossible to perform diagnostic evaluation because of sleep interruption). These different reasons for not completing the protocol require different approaches for correction.
The rate of missed hearing screenings was shown to increase significantly at the second screening and diagnostic evaluation. At this stage, the highest rate of non-compliance was observed in the group with risk factors who was classified as pass in screening. This could be justified by the fact that the result could somehow falsely reassure parents and health professionals.17 In addition, the time constraints of screening all children with risk factors by six months of age, as recommended at the time of the study, prioritizes children classified as refer in both screenings. This ensures a more timely diagnosis of children who are more likely to have hearing loss, but also delays the diagnostic evaluation of the remaining children to more advanced ages, resulting in the need for multiple diagnostic evaluations before a definitive result is obtained (due to greater difficulty with spontaneous sleep and higher incidence of middle ear effusion with age). Missed hearing screenings in this group of children may be related to parental difficulty in understanding that children with hearing loss may respond to auditory stimulation, depending on the severity of the hearing loss. Beyond this point, an altered screening result is sometimes attributed to transient CHL without recognizing the possibility of its coexistence with a sensorineural deficit.17-18 These points reinforce the need for ongoing professional training and parental awareness.
The discrepancy between the missed hearing screening rates in 2018 and previous years can be explained by the fact that data collection was completed in 2019, which may not have been sufficient to ensure adequate follow-up of children born in the previous year, thus affecting the results.
Regarding the UNHS program time targets, CHBV achieved the target of screening in the first month of life. However, the median time to diagnosis for refer cases was 6.5 months instead of the currently recommended 3 months. This highlights the missed opportunity to act at an ideal time window and reinforces the need to implement measures to reduce the rate of missed hearing screening at diagnostic assessment. This is also recognized as a reality internationally, as reported in a study where screening referrals failed to meet the recommended goal of assessment by six months of age in two-thirds of cases.17
When comparing the two screening techniques, the dissonance in results can be explained by the immaturity of the auditory pathways at the time of the first evaluation.
The CHBV computer platform allows several actions, including changing the category to which each child belongs (with vs. without risk factors) at any time, defining the incidence and prevalence of hearing loss in the population, and saving human and material resources. A future national online platform would be of great value to facilitate the monitoring of children followed in more than one hospital and in situations of change of residence.4
No less important, the presence of a multidisciplinary team is highly recommended for the success of a UNHS program.4
Conclusions
Universal newborn hearing screening is an essential tool for early diagnosis and prognosis of hearing loss. Stratifying infants according to the presence or absence of risk factors for hearing loss may be a valuable improvement to the current national protocol. First-degree parental consanguinity is an important risk factor in this population. Improving the rate of missed diagnostic screenings is a priority area for intervention.
Authorship
Bárbara Leal - Conceptualization, Data Curation, Formal Analysis, Writing - original draft, Writing - review & editing
Ana Cristina Lopes - Conceptualization, Data Curation; Validation; Writing - original draft
Daniela Peixoto - Data curation; Writing - original draft
Laura Correia - Data curation; Writing - original draft
Maria Miguel Almiro - Conceptualization; Writing - original draft; Supervision
João Vilar - Resources; Software
Luísa Azevedo - Supervision; Validation
Maria Adelaide Bicho - Conceptualization; Supervision; Validation















