Introduction
Hematuria is one of the most common urinary symptoms and can range from an incidental finding to a significant symptom requiring prompt treatment. Hematuria may be associated with various urinary tract disorders, trauma, or infection. The etiology can be difficult to determine and often requires multiple diagnostic approaches, including urinalysis, urine culture, urine protein quantification, renal function study, immunologic study, imaging study, cystoscopy to lateralize the bleeding source, and renal biopsy.1) Several vascular diseases, such as renal vein thrombosis, renal arteriovenous malformation, retroaortic left renal vein (LRV), and nutcracker syndrome (NCS), can cause macroscopic hematuria.
NCS, also known as left venous hypertension, was first described by Grant in 1937 and is a rare disease caused by compression of the LRV between the abdominal aorta and the superior mesenteric artery (SMA).2-4) The compression impairs blood flow through the LVR, creating a higher venous pressure gradient, resulting in renal venous hypertension and development of collateral circulation.5
The exact prevalence of NCS is unknown due to the lack of definitive diagnostic criteria and the wide variability of presentations.3It can be diagnosed at any age, with a greater prevalence of symptoms reported in the second to the third decades of life. The gender prevalence is unknown, although it may be slightly more common in women.5
NCS may remain asymptomatic, with patients presenting with a narrowed vein without an increase in pressure gradient, or it may manifest clinically with multiple symptoms related to the increased venous pressure caused by compression. Hematuria is the most common symptom, probably secondary to rupture of the thin-walled septum between the small veins and the collecting system in the renal fornix.6,7 Hematuria varies from micro to macrohematuria depending on the severity of LRV hypertension. The development of orthostatic proteinuria, which appears to be more common in puberty, may also be associated with an increased venous pressure gradient.8 Other less common manifestations include flank and pelvic pain and varicocele, reflecting renal and pelvic congestion.2,6,7
NCS is a diagnosis of exclusion, established through the use of several imaging modalities, including Doppler ultrasound (DUS), computed tomography angiography, magnetic resonance angiography, and retrograde venography. DUS is recommended as first-line study, as it is a non-invasive and inexpensive exam that can measure blood flow velocity and reveal the compression process caused by SMA.8,9
The management of NCS depends on the clinical presentation, age of the patient, and type of anatomical variant.
Case report
A 12-year-old girl was admitted to the hospital with macroscopic hematuria with 12 hours of evolution. She denied other urinary symptoms, such as dysuria, pollakiuria or urinary urgency, fever, or local trauma. The last menstrual period had occurred 15 days earlier. The girl reported a self-limited episode of fever and odynophagia, which was treated with paracetamol for three days. She had no relevant family or personal history. Menarche had occurred eight months earlier, and menstrual cycles were regular.
The girl weighed 46 kg, measured 160 cm (percentile (P) 73 of the WHO Child Growth Standards), and her body mass index (BMI) was 18 Kg/m2 (P40 of the WHO Child Growth Standards). She denied weight gain or loss. The physical examination was normal. Blood pressure was 112/62 mmHg (P50-90/P5-50 for age, sex, and height according to the update of the Fourth Report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents, 2017).
The urine dipstick showed mild proteinuria (2+, ≈100 mg/dl) and severe hematuria (4+, ≈200 uL), and the sediment showed 67/uL erythrocytes, 86/uL leukocytes, and no pathological cilindres. The protein/creatinine ratio was 0.50 mg/mg and the urine culture was negative. Urinalysis is depicted in Table 1. Blood tests were normal (Table 2), including renal function (creatinine 0.65 mg/dL, urea 26 mg/dL).
Table 1 Urianalysis on admission
| First morning urine sample | Value | Normal range |
| Creatinine | 67.42 mg/dL | 47 - 110 mg/dL |
| Total protein | 33.80 mg/dL | < 14 mg/dL |
| Protein/creatinine ratio | 0.50 mg/mg | < 0.20 mg/mg |
| 24-hour urine sample | ||
| Total protein | 375.30 mg/24h | < 300.00 mg/24h |
| Creatinine | 834.8 mg/24h | 950 - 2,490 mg/24h |
| Urinalysis | ||
| pH | 5.0 | 4.8 - 7.4 |
| Protein | 75 mg/dL | 0 - 10 mg/dL |
| Leukocytes | 25/uL | 0 - 10/uL |
| Erythrocytes | 250 /uL | 0 - 5/uL |
| Nitrites | Negative | |
| Sediment | ||
| Leukocytes | 86 /uL | 0 - 17/uL |
| Erythrocytes | 67 /uL | 0 - 23/uL |
| Bacteria | 22 /uL | 0 - 130/uL |
| Cilindres | 2/uL | |
| Urine culture | Negative |
Table 2 Blood analysis on admission
| Analytical parameter | Value | Normal range |
| Hemogram | ||
| Hematocrit | 39.2% | 36.0 - 46.0% |
| Hemoglobin | 13.7 g/dL | 12.0 - 16.0 g/dL |
| Leukocytes | 9120/uL | 5,000 - 13,000/uL |
| Platelet | 211,000 uL | 200,000 - 500,000/uL |
| Erythrocyte sedimentation rate | 5 mm/h | 2-20 mm/h |
| Biochemistry | ||
| Creatinine | 0.65 mg/dL | 0.57 - 1.11 mg/dL |
| Urea | 26 mg/dL | 15 - 36 mg/dL |
| Aspartate aminotransferase | 27 U/L | 5 - 34 U/L |
| Alanine aminotransferase | 15 U/L | < 55 U/L |
| C-reactive protein | 1.48 mg/dL | < 0.5 mg/dL |
| Immunologic study | ||
| Complement component C3 | 118 mg/dL | 71.87 - 122.03 mg/dL |
| Complement component C4 | 21.0 mg/dL | 9.92 - 29.96 mg/dL |
| Immunoglobulin A | 278 mg/dL | 71.20 - 434.12 mg/dL |
| Immunoglobulin G | 1080 mg/dL | 835 - 1716 mg/dL |
| Immunoglobulin M | 282 mg/dL | 24.29 - 198.69 mg/dL |
| Anti dsDNA | 9.8 UI/mL | negative: < 27 UI/mL |
| Anti-cromatin/nucleossome antibody | 1.0 U | negative: <20 U |
| Anticelular antibody | 0.30 U/mL | negative: < 0.7 U/mL |
| Anti- glomerular basement membrane | 20.1 U | negative < 20 U |
| Antigens related with myositis | 1-4 RU/mL | negative: 0-7 RU/mL |
| Serology test | ||
| Anti-streptolysin-O test | 208 UI/mL | < 200 UI/mL |
| Hepatitis B surface antigen | 0.16 | negative: < 1 |
| Hepatitis C antibody | 0.13 | negative: < 1 |
| Human immunodeficiency virus surface antigen | 0.1 | negative: < 1 |
| Coagulation study | ||
| Prothrombin time | 13.1 sec | 10.0 - 14.0 sec |
| Partial thromboplastin time | 30.1 sec | 25.1 - 36.5 sec |
| International normalized ratio | 1.10 |
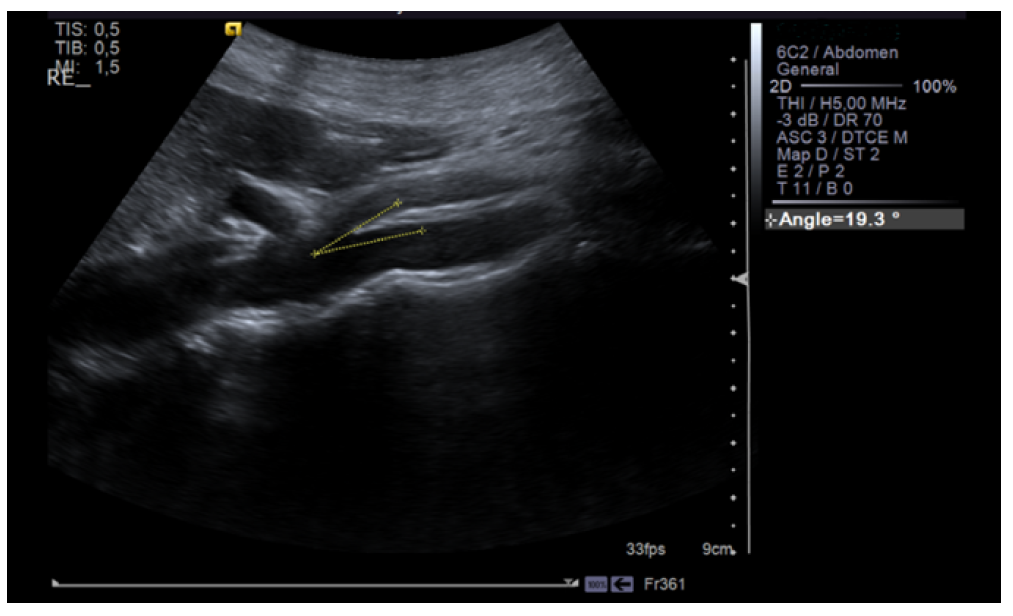
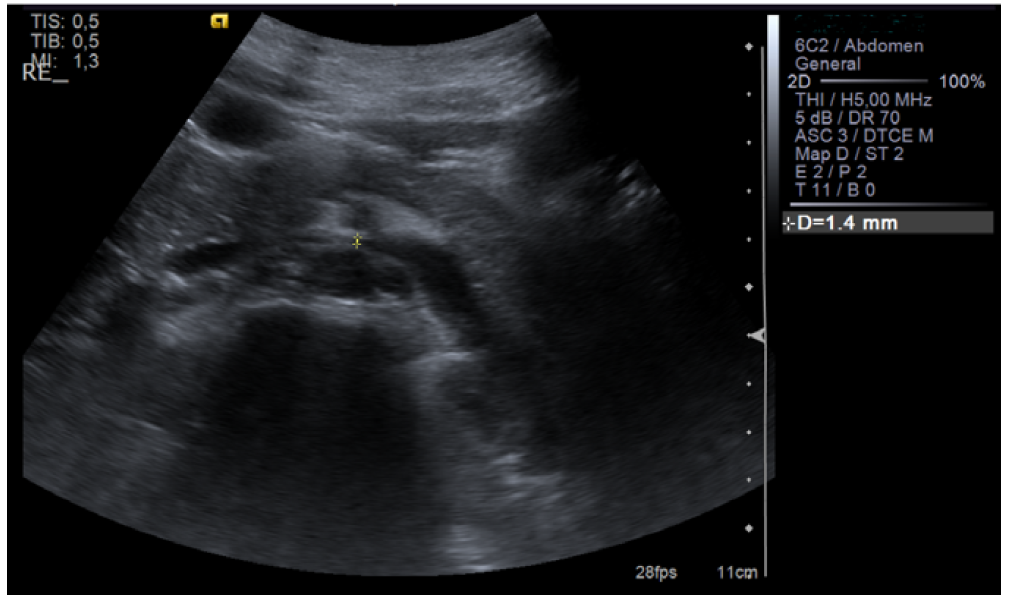
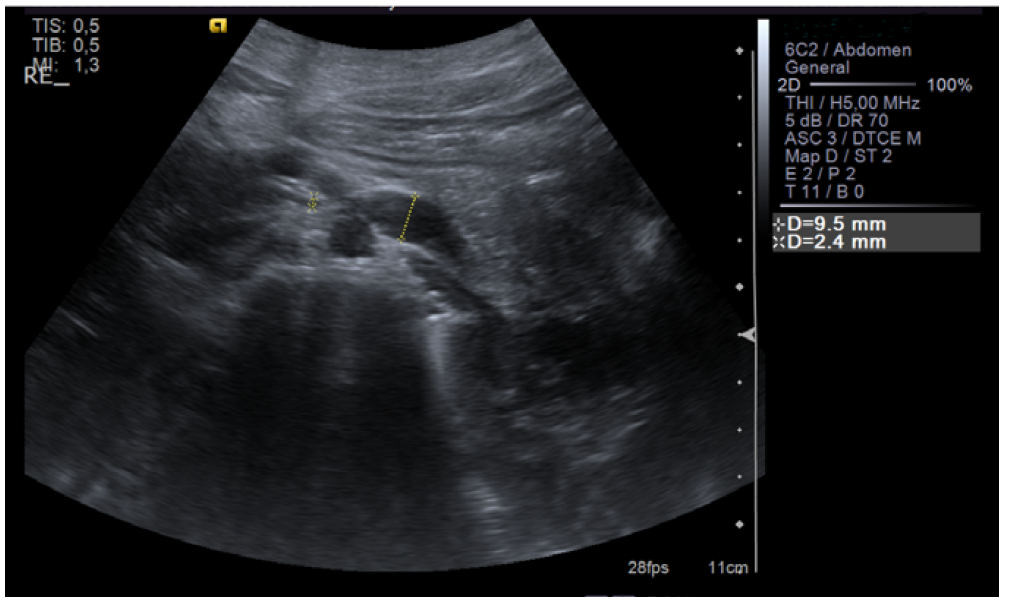
Renal ultrasound was performed and showed normal renal size and outline with no anatomic defect and preserved parenchymal thickness. No peri-renal fluid collections or signs of overt nephrolithiasis or dilatation of the excretory cavities were observed. After 10 days, since intermittent and asymptomatic gross hematuria persisted and trauma, hemoglobinuria, myoglobinuria, and renal and urinary tract pathology (urinary tract infection, glomerulopathy, tumor, nephrolithiasis) were excluded, renal vascular Doppler was performed, which showed “reduction of the aortomesenteric angle (19°), causing segmental reduction of the caliber of the LVR segment (1.4 mm in diameter), with increased velocity and turbulence, and dilation of the upstream vein (9.5 mm)”. These findings were consistent with NCS (Figures 1, 2, and 3).
The girl maintained macroscopic hematuria for two weeks. After discussing the case with a pediatric Nephrology unit at a tertiary center, conservative management with regular follow-up was decided. Two additional limited episodes of asymptomatic macroscopic hematuria lasting three days occurred one and two months after hospitalization. At the 12-month follow-up visit, the girl had only microscopic hematuria with no further episodes of macroscopic hematuria. She weighed 50.4 kg, measured 162 cm (P64 of the WHO Child Growth Standards), and had a BMI of 19.2 Kg/m2 (P50 of the WHP Child Growth Standards). Blood pressure was normal, and no proteinuria was detected in the first morning spot urine.
Discussion
NCS is a rare entity that refers to the extrinsic narrowing of the LRV between the abdominal aorta and the proximal superior mesenteric artery. The present case can be classified as anteriorNCS, one of the two anatomical variants of this syndrome. The posterior variant refers to the retroaortic position of the LRV, with compression of the vessel between the aorta and the vertebral column.3,5
NCS is a diagnosis of exclusion that should always be suspected when the presenting symptoms cannot be explained by other urinary tract or renal disorders, trauma, or infection. NCS is believed to be an underdiagnosed entity. Several imaging modalities can be used for diagnosis, including DUS, computed tomography, angiography, magnetic resonance angiography, and even phlebography. In this study, real-time DUS was used because it is a noninvasive exam with high sensitivity (about 80%) and specificity (almost 100%) for identifying LRV compression.3,8 Variations in the normal anatomy of the renal vasculature make the diagnosis challenging, and there are no fixed validated diagnostic criteria for the condition. However, some standard values are commonly used to guide the evaluation of renal vessels. In particular, when the SMA branches of the aorta are at an acute angle (usually less than 35°), the initial descent trajectory may cause LRV compression. Therefore, the LRV, usually 4-5 mm in diameter, shows a stenotic area in the aortomesenteric portion, dilation of the distal portion, and an increase in peak velocity at the aortomesenteric/renal hilum.8,10,11
Treatment of NCS is controversial and depends on the severity of symptoms. Non-surgical treatment is preferred in patients younger than 18 years with mild symptoms (microscopic hematuria and orthostatic proteinuria) and should be maintained for 24 months because the increase in intra-abdominal adipose tissue during growth may relieve the obstruction caused by SMA. Conservative management was considered appropriate for the patient in question, as she presented with mild symptoms. Dietary counseling was implemented to correct some dietary errors. A hypercaloric diet was not suggested in this case given the adequacy of body habitus and BMI.
The use of angiotensin-converting enzyme inhibitors to improve orthostatic proteinuria has been suggested in previous studies.8 In pediatric patients with symptoms persisting for more than 24 months or worsening, such as hematuria associated with anemia and severe pelvic or abdominal pain, a surgical approach may be considered. Several surgical techniques have been described, including open surgery (left renal autotransplantation, LRV transposition), laparoscopic surgery, and endovascular surgery, all with satisfactory results. However, due to the paucity of data, especially in the long term, it is difficult to determine the best approach for NCS.7,8,11 Open surgery has been considered the mainstay of treatment for NCS, the endovascular approach is gaining strength as more evidence becomes available.8,12
Conclusions
NCS is a rare cause of macroscopic hematuria. It is probably an underestimated disease because of the non-specific clinical findings and wide spectrum of symptoms. NCS is a diagnosis of exclusion and real-time DUS is recommended as the first-line diagnostic exam. Non-surgical treatment is preferred in patients younger than 18 years.
Authoship
Íris Santos Silva - Conceptualization; Investigation; Project Administration; Resources; Supervision; Validation; Visualization; Writing - original draft; Writing - review & editing
Joana Filipe Ribeiro - Investigation; Resources; Validation; Visualization; Writing - review & editing
João Virtuoso - Investigation; Resources; Validation; Visualization; Writing - review & editing
Rita S. Oliveira - Conceptualization; Project Administration; Resources; Validation; Visualization; Writing - review & editing
Glória Silva - Conceptualization; Project Administration; Resources; Validation; Visualization; Writing - review & editing
António Mendes - Conceptualization; Project Administration; Supervision; Validation; Visualization; Writing - review & editing