Introduction
Tuberculosis (TB) is a major global public health problem. The burden of pediatric TB is significant even in developed countries. In fact, a significant proportion of TB morbidity and mortality is reported in childhood, as children under the age of five are at increased risk of developing disseminated infection after primary TB, particularly miliary TB and meningitis.1,2 Disseminated forms result from the hematogenous spread of Mycobacterium tuberculosis and are classified as tuberculous infection involving the bloodstream, bone marrow, liver, or two or more noncontiguous sites.3 These severe extrapulmonary forms account for the high mortality rates seen in children. Late sequelae are also common, especially in cases with central nervous system involvement, and present as cranial nerve palsy, gait disturbances, hemiplegia, blindness, deafness, learning disabilities, and hypothalamic and pituitary dysfunction syndromes.4 In 2019, an estimated 205,000 children under the age of 15 years died of TB.5
In Portugal, a progressive and sustained reduction in the incidence of TB has been observed. In 2014, the threshold for Portugal to be considered a low-incidence country (<20/100000 inhabitants) was reached.6 Therefore, in 2016, the administration of the Bacillus Calmette-Guérin (BCG) vaccine in the country was restricted to risk groups, in accordance with the World Health Organization recommendations.7
Nationwide, risk groups are identified based on well-defined criteria for children under six years of age: coming from a country with a high incidence of tuberculosis; having completed the contact tracing process and/or prophylaxis/treatment scheme; having traveled to a country or living in a community with a high incidence of TB; or having parents or other cohabitants coming from a country with a high incidence of TB in the last ten years or with a history of HIV infection and/or alcohol or drug addiction. However, even in countries with a low TB burden, outbreaks remain a serious concern, particularly affecting vulnerable groups.2 The possibility of acquiring TB after occasional and brief contact with highly contagious patients not only adds to the increased susceptibility of this age group but also makes it difficult to rely on an epidemiologic link.8 These children serve as sentinel cases, indicating recent and/or ongoing transmission in the community.
In this report, the authors present the case of an infant with disseminated TB.
Case report
A previously healthy two-month-old female infant was admitted to the Pediatric Emergency Department with low-grade fever and mild nasal discharge with four days of evolution. Other respiratory or gastrointestinal symptoms were denied, and no epidemiologic link was identified. The girl was a term infant born by vaginal delivery at 40 weeks gestation. Birth weight was normal for gestational age. The BCG vaccine was not administered at birth because she did not meet national eligibility criteria. Her household was composed of the mother and father, and her socioeconomic status was middle class. Three years prior to pregnancy, the girl’s mother had been treated with rifampicin for latent TB infection (positive interferon-gamma release assay [IGRA]) after casual contact with a patient with untreated active respiratory TB). She had no symptoms during pregnancy or postpartum and routine antenatal screening and obstetric ultrasound were normal.
On admission, physical examination revealed a febrile (rectal temperature 38.6°C) but comfortable infant with no signs of respiratory distress and clear breath sounds. No palpable enlargement of the liver or spleen was noted.
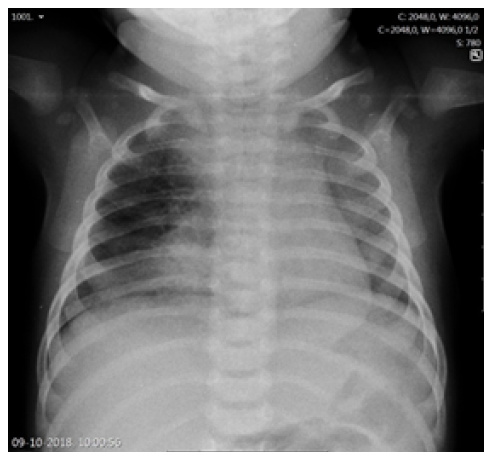
The duration of fever, young age, and subsequent immunization status (the girl had not yet received the two-month vaccines required by the national immunization program) prompted etiologic study, and a complete blood count revealed leukocytosis with neutrophilia (white blood cell count 29,300/uL, neutrophils 21,000/uL, lymphocytes 4,900/uL), hemoglobin of 10.1g/dL, and platelet count of 590,000/uL. C-reactive protein (CRP) was 47.2mg/L and procalcitonin (PCT) was 0.29 ng/mL. Altered coagulation study (international normalized ratio [INR] 1.45 and prothrombin time 16.7 seconds) were reversed after a single vitamin K administration. Serum electrolytes, renal function, and liver enzymes were normal. Chest radiograph showed a right apex and right perihilar infiltrate (Figure 1). Lumbar puncture was performed after clinical deterioration, and cerebrospinal fluid (CSF) analysis was normal (5/uL cells, 1/uL erythrocytes, glucose 54 mg/dL, proteins 0.42g/L).
Ampicillin and cefotaxime were administered empirically for suspected sepsis with bacterial pneumonia, with the goal of broad empirical coverage of major pathogens according to age group and immunization status.
Despite combination antibiotic therapy, the infant’s general condition deteriorated, with persistent high fever and consistent elevation of inflammatory markers (maximum erythrocyte sedimentation rate 76 mm/h, maximum CRP 130 mg/L). Vancomycin was added, but the fever persisted. Liver enzymes remained within normal range, without hypertriglyceridemia or hyperbilirubinemia. Other relevant laboratory findings included lactate dehydrogenase 343 U/L, ferritin 456 ng/dL, and anemia (nadir 8.6 g/dL), with no other cytopenias. Respiratory viral panel and bacterial cultures of blood, urine, and CSF were all negative. Human immunodeficiency virus serology was negative and echocardiogram and abdominal and renal ultrasounds were unremarkable.
After reevaluation of the chest radiograph, which remained unchanged, a computed tomography (CT) scan of the chest showed multiple adenopathies in the middle mediastinum compressing the trachea, right main bronchus, and venous vascular structures, with ground-glass opacities in the anterior segment of the right upper lobe, in anatomic correlation with the airway obstruction (Figure 2).
Given the lack of response to antibiotic therapy after five days (total of ten days of fever) and CT findings, the diagnosis of TB was considered. Gastric aspirate (GA) specimens were obtained, and bronchoscopy revealed compression of the trachea and right main bronchus, but no endobronchial lesions.
After positive acid-fast bacilli (AFB) smear from bronchoalveolar lavage (BAL) and gastric aspirates (GA), therapy with isoniazid, rifampicin, pyrazinamide, and ethambutol (HRZE) was initiated. Polymerase chain reaction (PCR) was used to detect M. tuberculosis in BAL and GA specimens and was positive in three GA and BAL and one urine sample (collected to assess for possible systemic dissemination). M. tuberculosis was sensitive to all four tuberculostatic agents. CSF PCR and culture were negative. No hepatic or splenic granulomas were found. Transfontanellar ultrasound and ophthalmologic examination were normal and IGRA was positive.
After 14 days of fever, sustained apyrexia was achieved with 48 hours of HRZE. The girl’s clinical condition rapidly improved, and she was discharged after 22 days of HRZE, asymptomatic and with optimal weight gain. As disseminated infection was confirmed by a positive M. tuberculosis urine culture, the girl completed 12 months of HRZ combination therapy. Adequate multidisciplinary follow-up was ensured, and no long-term sequelae were identified.
TB screening was performed on all close family members and other contacts considered relevant. Both parents and paternal grandparents performed chest radiography, as did a friend couple with close contact with the family. Their 12-month-old son had a tuberculin skin test followed by IGRA, which were negative, and a chest radiograph that was also normal. The girl’s mother further performed a chest CT, which showed no abnormalities. Five healthcare workers with close contact with the infant also underwent TB screening. Extensive contact tracing failed to identify the index case.
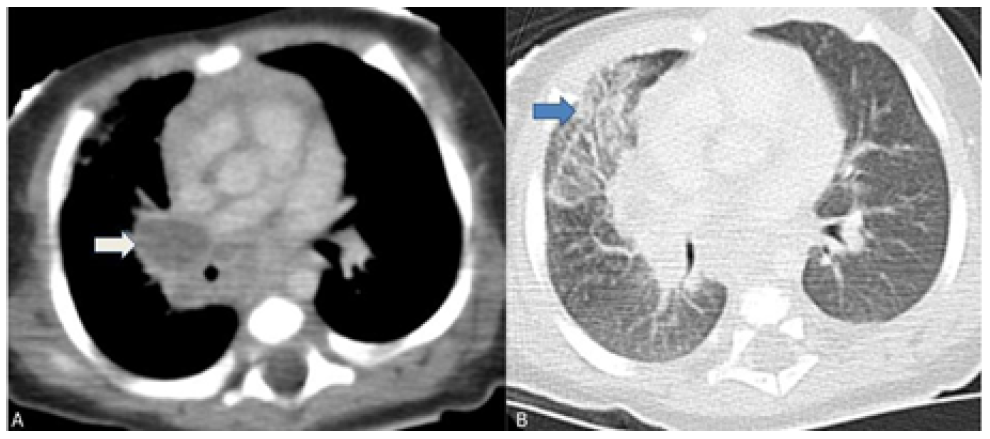
Figure 2 Chest computed tomography scan showing multiple mediastinal adenopathies causing external airway compression. (A) Right hilar adenopathies compressing the right main bronchus (white arrow); (B) Ground glass opacity of the anterior segment of the right upper lobe indicating atelectasis (blue arrow).
Discussion
The diagnosis of pediatric TB is challenging, especially in infants, as seen in the present case report. In this age group, TB often progresses rapidly from infection to disease, with a much higher incidence of disseminated forms than in other age groups. Delayed diagnosis is directly correlated with higher morbidity and mortality. Several factors are recognized as contributing to the pediatric disease burden paradigm, the most frequently cited being the heterogeneity and nonspecificity of signs and symptoms.9,10 The difficulty of obtaining microbiologic specimens is also relevant, as intermittent positivity and paucibacillary disease often require multiple specimens.11 Gastric aspiration is the preferred method for obtaining respiratory specimens from young children.1
Children’s vulnerability to the disease is further supported by evidence that brief exposure to highly contagious patients may be sufficient to cause early and severe disseminated disease.7 Although the clinical history remains the cornerstone of diagnosis, the absence of an obvious epidemiologic link should not delay investigation in face of the clinical suspicion that should arise in any atypical respiratory illness. Therefore, it is of utmost importance to raise awareness of pediatric forms of TB, not only to ensure early detection and treatment, but also because children act as sentinel cases, indicating recent and/or ongoing transmission within the community. Confirmation of the disease in children should prompt immediate contact screening seeking to interrupt the chain of transmission. However, given the rapid pathophysiologic course of infection in children, identification of the index case can be particularly challenging, as demonstrated in this case. In addition, children are excellent indicators for monitoring the effectiveness of control measures and for targeting at-risk groups for timely vaccination.12
Regarding pulmonary and thoracic imaging in children, chest radiography often shows opacity with minimal parenchymal involvement that correlates with the primary complex. However, airway obstruction (e.g., due to lymphadenopathy) may result in consolidation or segmental lesion, leading to the collapse of a lung segment or lobe in the setting of infiltrate and atelectasis, as illustrated in this case.13 The role of chest CT should also be emphasized, as it is a very useful tool in cases of clinical and radiologic discrepancy, accurately accessing mediastinal ganglia involvement and airway compression, and helping to exclude other pathologies.14 Despite being an invasive exam, bronchoscopy should be performed to better characterize airway compression and/or endobronchial lesions and when there is a high clinical suspicion despite negative microbiological results or when treatment response is inadequate.
In conclusion, this report describes a case of disseminated TB in an infant with few respiratory symptoms and no identifiable epidemiologic link, emphasizing the importance of a high index of suspicion, especially in this age group, where infection can occur with little exposure. TB infection should be suspected in any child with unresponsive worsening pneumonia, and a full workup including extrapulmonary forms should be performed.
Authorship
Margarida S. Abreu - Conceptualization; Investigation; Methodology; Supervision; Validation; Visualization; Writing - original draft; Writing - review & editing
Sofia Miranda - Investigation; Methodology; Writing - original draft
Isabel Carvalho - Formal analysis; Supervision; Validation; Visualization; Writing - original draft; writing - review & editing
Augusta Gonçalves - Conceptualization; Formal analysis; Methodology; Validation; Writing -original draft; Writing- review & editing
Liliana Branco - Validation; Writing - original draft; Writing - review & editing
Daniela Barros - Resources; Visualization; Writing - original draft; Writing - review & editing
Marina Pinheiro - Formal analysis; Visualization; Writing - review & editing